
Mifepristone case brings abortion access back before the US Supreme Court
The justices must decide whether to increase restrictions on drug that is used in more than half of all pregnancy terminations in the country

The justices must decide whether to increase restrictions on drug that is used in more than half of all pregnancy terminations in the country

The central dispute in the case is whether the Food and Drug Administration overlooked serious safety problems when it made mifepristone easier to obtain, including through mail-order pharmacies

A legislative reform being prepared by the European Union plans to promote the use of already approved drugs to treat other illnesses. Doing so will speed up the approval of treatments and save on research costs

After half-a-century of prohibition, the United States is about to legalize psilocybin and MDMA for clinical use, to treat people suffering from post-traumatic stress disorder or terminal cancer. Scientists, patients, clandestine therapists and war veterans speak with EL PAÍS about the bright spots and shadows of this rebirth

The controversial entrepreneur announced his company’s product will be called Telepathy, and will allow patients to control a cell phone and a computer with their mind

The economic impact of obesity will reach losses of up to $4 trillion by 20235, or 3% of global GDP
Some experts worry the U.S. may be repeating mistakes that gave rise to the opioid crisis: overprescribing a questionable drug that carries significant safety and abuse risks

The experts consulted see the approval of the use of zuranolone as good news, but they also point out that we must not lose focus on the many other aspects that surround a mother and her baby

The panel of Food and Drug Administration experts met Wednesday to review a stem cell-based therapy that has been at the center of a yearslong lobbying campaign by patients
The tycoon’s biotech company is currently looking for human volunteers to test its implants, which have allowed animals to control external devices with their minds
A team of scientists has managed to prevent neuronal death with oral drugs administered to rodents that have been genetically modified to imitate dementia
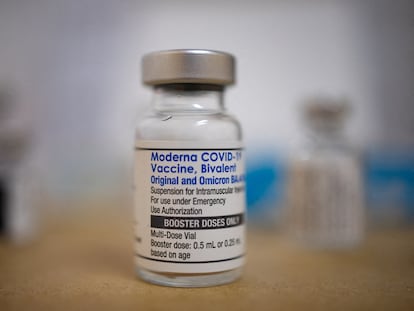
The Food and Drug Administration decision opens the newest shots from Moderna and Pfizer and its partner BioNTech to most Americans even if they’ve never had a coronavirus vaccination

The decision by three judges on the 5th U.S. Circuit Court of Appeals in New Orleans overturned part of a lower court ruling that would have revoked the Food and Drug Administration’s 23-year-old approval of mifepristone

All of the recalled cookies, soup and falafel have been removed from sale or destroyed, Trader Joe’s said in its announcements

While the USDA just approved two companies that produce lab-grown meat, Italy, as a precaution, banned its production and sale
The Food and Drug Administration on Monday approved the injection for infants and children up to 2 years old. It’s made by AstraZeneca and is already approved in Canada and Europe

Medical societies and women’s health groups have pushed for wider access for decades, noting that an estimated 45% of the 6 million annual pregnancies in the U.S. are unintended
The decision means that the treatment, which modestly slows down the advance of the brain-robbing disease, should be covered under the U.S. health insurance program

The number of different electronic cigarette devices sold in the U.S. has nearly tripled to over 9,000 since 2020, driven almost entirely by a wave of unauthorized disposable vapes from China

The Food and Drug Administration’s scientific advisers said the next round of shots in the U.S. should only include protection against the newest variants that are now dominant worldwide
The decision carries extra significance because insurers have held off on paying for the drug, Leqembi, until it has full FDA approval

The drugmaker is seeking to halt the program, which was laid out in the Inflation Reduction Act and is expected to save taxpayers billions of dollars in the coming years

The drug is similar to naloxone, the life-saving drug that has been used for decades to quickly reverse overdoses caused by opioids

The Food and Drug Administration asked if there’s a hint that the vaccine might affect premature birth but its advisers weren’t convinced
The conservatives judges of the New Orleans-based 5th U.S. Circuit Court of Appeal will hear arguments May 17 on whether the FDA’s approval of mifepristone must be rolled back

Even though nearly half of states already allow canine dining outdoors, the issue is far from settled, with many diners and restaurants pushing back against the increasing presence of pooches

The recommendation came at the close of a two-day meeting focused on whether women could safely and effectively take the pill without professional supervision