Gene-editing ‘pencil’ saves two teenagers dying from cancer, putting their leukemia in remission
Sadly, a third young man died in the clinical trial. It was conducted at a London hospital that receives funding from royalties earned from the book ‘Peter Pan’
It’s one of the most fascinating stories in medicine. In 2003, Spanish researcher Francis Mojica accidentally discovered that certain microbes from salt flats use molecular scissors to eliminate invading viruses, chopping up their genetic material. Then, in 2012, American chemist Jennifer Doudna and French biochemist Emmanuelle Charpentier realized that these microbial scissors – named CRISPR – could be used to modify DNA. They ended up winning the Nobel Prize for this discovery. And, in 2016, American chemist David Liu invented base-editing – the second generation of CRISPR tools – which acts like a pencil with an eraser, capable of erasing a single letter from DNA and replacing it with another.
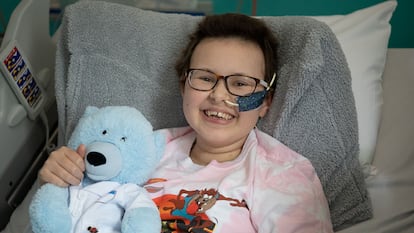
A year ago, Alyssa – a 13-year-old British teenager with a very aggressive form of leukemia – became the first person to benefit from this technology. Today, Alyssa is back at home: her cancer is in complete remission. This past Wednesday, the scientists involved published the details of her case, along with those of two other young people.
The three patients were doomed to die from T-type acute lymphoblastic leukemia – a blood cancer that was not responding to either chemotherapy or bone marrow transplants. The medical team – led by Waseem Qasim, from University College London – resorted to the revolutionary CAR-T treatments. This therapy consists of extracting white blood cells from patients, redesigning them in a lab to multiply their ability to destroy cancer cells and re-introducing them into patients. In this case, the researchers used donor white blood cells, but used base-editing – the pencil with an eraser – to turn them into universal super white blood cells.
Qasim’s team announced their success with Alyssa in December, at the American Society of Hematology’s annual meeting. And, this past Wednesday, the researchers revealed the existence of two other anonymous patients: two boys who were 15 and 13- year-old when they were diagnosed. The treatment achieved the “deep remission” of the 15-year-old’s leukemia in just one month. The third case, on the other hand, was tragic. The 13-year-old responded to CAR-T therapy, but suffered a runaway infection of the fungus Aspergillus niger in his lungs and died, according to results published in The New England Journal of Medicine.
The American immunologist Carl June – the father of CAR-T treatments – has compared some of the latest results with biblical resurrections, “like that of Lazarus,” according to what he said in an interview in EL PAÍS this past September. Since 2010, complete remissions of blood cancers – leukemias, myelomas and lymphomas – number in the hundreds. However, there are still problems to resolve, as emphasized by immunologist Jordi Minguillón, from La Paz hospital in Madrid. One of them is that the therapies also destroy healthy cells in the immune system, lowering a patient’s defenses. “Patients can get significant infections soon after receiving treatment – these can be very serious. This is one of the potential disadvantages of this therapy,” he warns. Price is another barrier. Pharmaceutical companies charge more than $300,000 for each patient treated with CAR-T, although public hospitals are striving to develop cheaper alternatives.

Treatments for the three teenagers have been carried out at London’s Great Ormond Street Children’s Hospital, which is partly funded by the royalties earned from Peter Pan, since its creator – Scottish novelist James Matthew Barrie – donated the rights to the institution in 1929. The goal of the researchers is to complete the clinical trial with a dozen patients, who are between the ages of six months and 16-years-old. Acute T lymphoblastic leukemia is a very rare disease – barely 100 cases are diagnosed each year in Spain, according to figures from the Josep Carreras Leukemia Research Institute. For patients who relapse after bone marrow transplants, the prognosis is very poor, with a long-term survival rate of less than 15%.
The therapy that the three adolescents underwent is a sample of the new frontier of medicine. Researchers have used a virus to introduce a receptor into donor white blood cells with an affinity for cancer cells. Scientists have then used the gene-editing pencil to modify three genes in the white blood cells, with three goals: prevent them from killing each other, make them resistant to parallel cancer treatments and make them suitable for donation. Thanks to these modifications, super white blood cells can identify cancer cells and kill them.
Alyssa would have passed away if it wasn’t for base-editingLluís Montoliu, geneticist
La Paz hospital in Madrid began its CAR-T program for children and adolescents in 2020, using white blood cells from the patients themselves. Since then, the hospital has had about 30 of these young cancer patients, especially those with another type of leukemia – type B – according to Dr. Minguillón. Survival rates exceed 50% even in the most experimental of therapies, but the immunologist is cautious. “We’re very hopeful, but this is like running a marathon. You can start with record speed, but you have to finish the race,” he emphasizes. Cancer cells are often left behind in the body, which can lead to a relapse. Minguillón points out that the great advantage of the London trial is that it allows the use of donor white blood cells – a decisive factor when patients are very sick and immunocompromised. The treatment is a bridge to a new bone marrow transplant, which – due to the red blood cells that are produced there – can rebuild the body’s defenses.
Geneticist Lluís Montoliu – from the Spanish National Center for Biotechnology – is astonished by the speed of the results. “David Liu launched base-editing in 2016. Alyssa was cured by them in May of 2022. Only six years [after the therapy was invented]! It’s a milestone!” the researcher celebrates. He points out that Waseem Qasim’s team could have used the first-generation CRISPR tools (scissors) to modify white blood cells, but Liu’s gene-editing pencil has enabled a precision that avoids dangerous DNA collateral damage.
“Alyssa would have passed away if it wasn’t for base-editing. And the jump is barely six years from when an idea came out of nowhere, until that idea has become a reality. Usually, in biomedicine, 10 or 20 years need to pass [for this to happen]. It’s incredible to me,” Montoliu sighs. His team is already using base-editing to try to transform some cells in the retina of mice who suffer from albinism, associated with a loss of visual acuity.
David Liu – from Harvard University – is co-founder of a company called Beam Therapeutics, which launched a trial in November focusing on patients who suffer from sickle cell anemia, a serious inherited disorder of the red blood cells. Another American company – Verve Therapeutics – has been experimentally using CRISPR base editors since July to deactivate genes that are associated with high levels of bad cholesterol. This technique has already achieved very promising results in mice, correcting common heart conditions. Montoliu has no doubt: “[CRISPR] base editors are here to stay. They’re going to triumph in the clinical trials.”
Sign up for our weekly newsletter to get more English-language news coverage from EL PAÍS USA Edition
Tu suscripción se está usando en otro dispositivo
¿Quieres añadir otro usuario a tu suscripción?
Si continúas leyendo en este dispositivo, no se podrá leer en el otro.
FlechaTu suscripción se está usando en otro dispositivo y solo puedes acceder a EL PAÍS desde un dispositivo a la vez.
Si quieres compartir tu cuenta, cambia tu suscripción a la modalidad Premium, así podrás añadir otro usuario. Cada uno accederá con su propia cuenta de email, lo que os permitirá personalizar vuestra experiencia en EL PAÍS.
¿Tienes una suscripción de empresa? Accede aquí para contratar más cuentas.
En el caso de no saber quién está usando tu cuenta, te recomendamos cambiar tu contraseña aquí.
Si decides continuar compartiendo tu cuenta, este mensaje se mostrará en tu dispositivo y en el de la otra persona que está usando tu cuenta de forma indefinida, afectando a tu experiencia de lectura. Puedes consultar aquí los términos y condiciones de la suscripción digital.









































