David Liu, chemist: ‘We now have the technology to correct misspellings in our DNA that cause known genetic diseases’
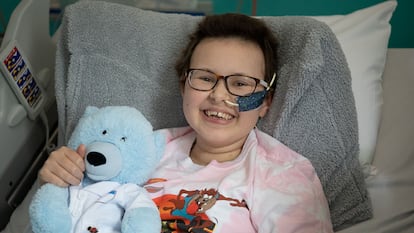
The American scientist has invented a revolutionary tool that can modify the human genome. It has already saved the life of a girl with a very aggressive type of cancer

A couple of decades ago, Harvard Magazine published a note about one of the university’s professors. Chemist David Liu had been banned from Las Vegas casinos when he was 29-years-old, after winning too much money playing blackjack. When asked if this is an urban legend, Liu smiles. “It’s partially true. Actually, I was 21-years-old… and it wasn’t all in one night,” he responds between laughs. The chemist was a young prodigy. At the age of 26, he was a lecturer at Harvard. By 31, he was already a full professor. For fun, he used his knowledge of math to gain an advantage at blackjack.
In 2016—when he was 43—his team had invented single-letter base editing: a tool used to modify DNA that is currently revolutionizing the world of medicine. Three months ago, a London hospital announced that it had used base editing to save the life of Alyssa: a 13-year-old girl with a very aggressive form of leukemia. “Her cancer is in complete remission,” Liu gushes.
The operating manual of a human being—present in every cell—is a piece of text with more than three billion chemical letters. Errors in this DNA cause cancer and a multitude of diseases. Liu wants to rewrite this human book to eliminate typos.
The Californian chemist, born in Riverside 49 years ago, compares his base editing tool to a pencil with an eraser, capable of removing a single letter and replacing it with another. Alyssa’s medical team, from University College London, used base editing to modify a donor’s white blood cells, to help them attack her cancer cells. David Liu’s amazing techniques have outdated previous gene-editing tools, including CRISPR, which was invented in 2012 and won the 2020 Nobel Prize in Chemistry. The researcher likens the original CRISPR to a pair of scissors: useful for deactivating genes in a rough way, but not rewriting them accurately.
Today, his own pencil with an eraser is already being surpassed. In 2019, Liu announced a new tool: quality editing. “It’s like a word processor: you can search for a specific sequence and replace the entire sequence with another sequence that you want,” he explains via videoconference. Quality editors—which are still in the experimental phase—can theoretically correct 89% of the 75,000 genetic variants associated with diseases.
Question. There are 20 million new cases of cancer every year around the world. How many of these patients could benefit from base editing or quality editing?
Answer. Well, cancer isn’t just one disease—it’s hundreds of diseases. And those diseases are each made up of many different kinds of molecular changes that cause cancer. I think the strategy used with Alyssa holds great promise for patients with T-cell leukemia and possibly other blood tumors… but it’s too early to tell what role these tools might play in other cancers.
Q. In 2016, this technology must have seemed like science fiction, even to you.
A. These database editors and quality editors don’t exist in nature. They are engineered molecular machines. It’s still kind of amazing to me that humans are taking control of our genomes—and the misspellings in our genomes—that cause genetic diseases.
Q. There are 400 million people affected by one of the 7,000 diseases caused by mutations in a single gene. Three months ago, your colleague Fyodor Urnov—from the University of California at Berkeley—wondered: “Why aren’t we curing them already?” He maintains that the main obstacles aren’t technical, but legal, financial and organizational.
A. I agree. There are still significant technical and scientific challenges, such as learning to modify DNA in ways that would be therapeutic. And, of course, we still don’t know how to [access] certain tissues in the body. But I agree with Fyodor in that there are manufacturing, regulatory and other non-scientific barriers that will need to be addressed if we want to maximize the benefit of these technologies in society.
Q. Are many people going to die because of obstacles that aren’t scientific?
A. If someone dies from a disease, it’s the fault of the disease, not the regulatory bodies. Regulators don’t kill anyone. The goal is to ensure that these treatments are as effective as possible, but also that they’re safe. The history of medicine is littered with examples where well-intended doctors and scientists didn’t understand the side effects of their experimental therapies well enough and ended up harming patients. The goal is to avoid doing that.
Q. How many letters do you receive from parents who have children living with genetic diseases?
A. About five or ten letters each week. We try to answer all of them. In the early days of base editing, technology could fix a few of the single letter mutations we were told about… But now, there’s almost always a technology to fix the bug, either with base editors or quality editors. In some cases, however, it hasn’t been shown that correcting the error can actually cure the patient. In some genetic diseases, it’s too late, because the damage appears very early. In many cases, unfortunately, I have to explain to the people who send us letters that it takes very solid science to link a genetic mutation to a disease. And you also need good animal models that mimic that disease. For most of these diseases, [these options aren’t available], so it’s hard to prove whether gene-editing can work. And of course, even if there are animal models, it takes years of work to show that correcting a mutation can correct the disease in a human.
I understand that it can be frustrating for a patient’s family to know that we know of a technology that can correct an error in DNA that could be the cause of the genetic disease affecting their son or daughter. However, gene-editing technology isn’t enough on its own. The good news is that we already have technologies that can correct the vast majority of DNA errors that cause known genetic diseases. But, while this is an important step, we also need all of these other pieces to fit together in order to develop therapeutic strategies that merit clinical trials and, hopefully, new drugs.
Q. In a New York Times article, Fyodor Urnov estimated that it takes four years and about $10 million to develop a gene-editing drug.
A. $10 million sounds about right. I would say it can cost between a million and $100 million dollars. And four years seems very fast to me. It seems like an ambitious deadline to start a clinical trial, but not to obtain the authorization of a drug. But, you know, that’s an important point for the public to appreciate. When they read a news article that says, “here’s a [research] paper showing that this new treatment can treat this disease in an animal,” this still means that there are probably years of work before such a treatment might be available to a patient.
Q. Who can invest $10 million to develop a drug that can only be used in a person with a specific mutation?
A. That’s one of the main issues facing our field. We’re already working on some strategies to try to solve this problem. I think there are a few ways that a gene-editing agent can be used to treat many different mutations and even many different genetic diseases. I hope we can report some good news about it soon.
Q. Now that Alyssa’s cancer has gone into remission, what’s next?
A. There are four ongoing clinical trials with base editing. One of them is that of Alyssa, from University College London. The first trial to administer base editors directly into patients (rather than into their cells in a laboratory environment) is a collaboration between the companies Verve and Beam, to reduce super high levels of bad cholesterol related to the PCSK9 gene. The Beam-101 trial—against sickle cell disease and beta thalassemia (a blood disorder that reduces the production of hemoglobin)—is already enrolling patients. And there’s also another clinical trial underway in China to treat beta thalassemia. I hope that Alyssa is the prelude to many more positive results.
Q. When will the quality editors be tested in a human clinical trial?
A. You would have to ask Prime Medicine (one of the companies that Liu co-founded, which is developing treatments with quality publishers). They hope to have an IND (investigational new drug) cleared by 2024. Hopefully clinical trials will begin soon.
Q. How do you imagine medicine in 10 years?
A. I would be disappointed if, 10 years from now, we didn’t have enough clinical trials, both with database publishers and quality publishers. And I hope we’ll have the first drugs approved that are molecular machines capable of going into a patient’s cell and specifically changing a bug [that’s causing] a genetic disease.
I hope that, if you interview me in 2033, there will be quite a few clinical trials and even some of the first FDA-approved drugs that allows us to take control of our genomes and not be so beholden to the misspellings in our DNA that determine the genetic fates of millions of people.
Q. You tweeted the other day that people are “happy accidents.”
A. I was just making the point that evolution is dependent on chance events. Organisms evolve and their mutations are partially random. For a gene to evolve one way or another can be considered a happy accident. Evolution depends on chance events that make the results stochastic—difficult to predict. In that sense, the fact that humans have evolved as they have is a bit of a matter of luck, or bad luck, depending on one’s perspective.
Q. You don’t see God’s hand in any aspect of DNA?
A. Well, that’s a tough question to answer. No comment! (laughs)
Q. You supported a moratorium on germline gene-editing, which involves altering the specific genes of an egg, sperm cell, or early embryo. That was back in 2019. Do you still support the moratorium?
A. It’s not a simple black and white issue, as it is with most important issues. I think there’s very little reason to edit the germline. I do anticipate that there will be more cases in the future, especially when somatic cell-editing [cells that are neither eggs nor sperm cells] has been tried more and there are approved drugs. That might be a better time to discuss the pros and cons of germline gene-editing. For now, I think the ethical and scientific stakes are too high to justify the small number of hypothetical cases in which [germline gene-editing] might be considered necessary.
Q. What kind of cases do you envision in the future?
A. Well, in the future there may be more willingness to edit the germline. Once somatic cell gene-editing is mature enough and there’s a high degree of confidence in its safety and efficacy, I think people will take another look at germline editing. Currently, I don’t think it should be a priority.
Sign up for our weekly newsletter to get more English-language news coverage from EL PAÍS USA Edition
Tu suscripción se está usando en otro dispositivo
¿Quieres añadir otro usuario a tu suscripción?
Si continúas leyendo en este dispositivo, no se podrá leer en el otro.
FlechaTu suscripción se está usando en otro dispositivo y solo puedes acceder a EL PAÍS desde un dispositivo a la vez.
Si quieres compartir tu cuenta, cambia tu suscripción a la modalidad Premium, así podrás añadir otro usuario. Cada uno accederá con su propia cuenta de email, lo que os permitirá personalizar vuestra experiencia en EL PAÍS.
¿Tienes una suscripción de empresa? Accede aquí para contratar más cuentas.
En el caso de no saber quién está usando tu cuenta, te recomendamos cambiar tu contraseña aquí.
Si decides continuar compartiendo tu cuenta, este mensaje se mostrará en tu dispositivo y en el de la otra persona que está usando tu cuenta de forma indefinida, afectando a tu experiencia de lectura. Puedes consultar aquí los términos y condiciones de la suscripción digital.









































