The Mpemba effect: When hot water freezes faster than cold
This phenomenon, which can be observed in a recent social media trend, remains a mystery to science

The Mpemba effect is a phenomenon through which, under certain conditions, a hot liquid can freeze faster than a cold one. This counterintuitive fact has been known at least since the time of Aristotle, approximately 2,300 years ago; more recently, it was rediscovered by Erasto Mpemba, a Tanzanian high school student, who, together with physicist Denis Osborne, first investigated it in the 1960s. Their paper, published in Physics Education in 1969, was appropriately titled “Cool?” A few months ago, a trend consisting of throwing boiling water into the air to see it quickly freeze and turn into snow went viral on social media.
@thecathasbread Hot water freezes faster than cold water #mpembaeffect #water #ice #freezes #fact #facts
♬ original sound - Lime
There has been some controversy regarding this phenomenon, as consistently replicating the result in the laboratory has proven difficult. The smallest details matter a great deal, such as the size, shape, and material of the container — even where the thermometer is placed. Various explanations have been proposed for the Mpemba effect: convection, evaporation, overcooling, impurities in the water, dissolved gases, and more. However, there is no commonly accepted argument, as all of the aforementioned phenomena seem to play a role.
Furthermore, while these explanations may be partially true in the case of water, they do not apply to other substances, such as magnetoresistance alloys, polymer alloys and granular systems, where the phenomenon is also observed. A 2017 study offers a general theoretical explanation using the theory of non-equilibrium thermodynamics: according to this theory, any point in the phase space of a fluid in equilibrium can be described by three numbers: its temperature, volume and number of particles. However, when the fluid is in the process of cooling, it is not in equilibrium and the number of states required to describe the system increases to infinite dimensions, so infinite numbers are needed to precisely quantify the state of the fluid.
When a hot liquid is placed in a cold environment, it tries to reach its lowest energy state. However, the energy landscape defined in its states has multiple local minima — that is, points that take lower values than those around them — called metastable energy wells. If a hot liquid enters these metastable energy wells, it has more energy to escape more easily and find the global minimum — the cooling temperature — whereas if a cooler liquid enters one of the metastable energy wells, it will spend more time there.
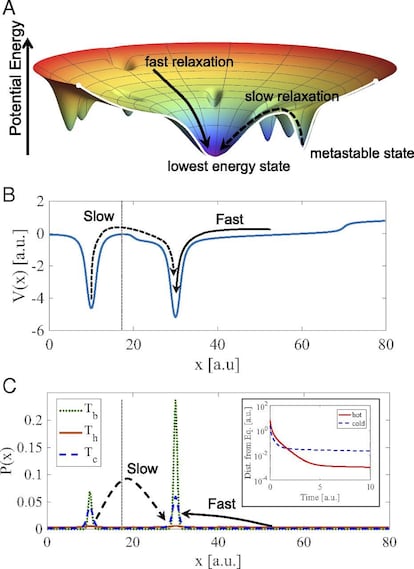
With greater precision, the state of the system can be modeled by means of a probability distribution function (PDF), which describes the probabilities of all the possible states that it could have, whose evolution is governed by a linear differential equation. For a system with finite states, this evolution is determined by the properties of the so-called transition matrix. Now, for this type of system, whatever the initial probability distribution is, it will, at some point, converge to the state of equilibrium. However, due to the shape of the transition matrix, there is a special PDF that will converge to the state of equilibrium at the slowest possible pace, compared to all other initial probability distributions.
Thus, to observe the Mpemba effect, we need the distance from the PDF to the state of equilibrium to be smaller for the hotter liquid, compared to the colder liquid, after some time. This can happen if, initially, the PDF for the colder liquid is closer to this special probability distribution than the one for the warmer liquid. This will result in the cooler liquid converging to the state of equilibrium more slowly than the warmer liquid, after some time.
Using this analysis, the authors of the paper predicted a reverse Mpemba effect in which, when two liquids heat up, the colder one may heat up faster. This phenomenon has now been observed in experiments. In a 2019 theoretical study where a similar approach was used, physicists also predicted a strong Mpemba effect in which, under carefully selected parameters, the hottest liquid can cool exponentially faster compared to the initial cold liquid. This has also been observed in experiments.
However, the Mpemba effect for water remains unresolved; we will have to wait a few more years for a definitive answer to this question.
Siddhant Govardhan Agrawal is a postdoctoral researcher at the Institute of Mathematical Sciences, in Spain.
Editing and coordination: Ágata A. Timón G Longoria (ICMAT).
Sign up for our weekly newsletter to get more English-language news coverage from EL PAÍS USA Edition
Tu suscripción se está usando en otro dispositivo
¿Quieres añadir otro usuario a tu suscripción?
Si continúas leyendo en este dispositivo, no se podrá leer en el otro.
FlechaTu suscripción se está usando en otro dispositivo y solo puedes acceder a EL PAÍS desde un dispositivo a la vez.
Si quieres compartir tu cuenta, cambia tu suscripción a la modalidad Premium, así podrás añadir otro usuario. Cada uno accederá con su propia cuenta de email, lo que os permitirá personalizar vuestra experiencia en EL PAÍS.
¿Tienes una suscripción de empresa? Accede aquí para contratar más cuentas.
En el caso de no saber quién está usando tu cuenta, te recomendamos cambiar tu contraseña aquí.
Si decides continuar compartiendo tu cuenta, este mensaje se mostrará en tu dispositivo y en el de la otra persona que está usando tu cuenta de forma indefinida, afectando a tu experiencia de lectura. Puedes consultar aquí los términos y condiciones de la suscripción digital.








































