Human pain, literally served on a plate
A scientific team has used millions of human cells to build neural circuits in the laboratory that sense painful stimuli and trigger suffering
A 39-year-old Spaniard — Santiago Ramón y Cajal — changed the history of science with a study hidden in the little-known Barcelona Journal of Medical Science, published on November 25, 1891. At a time when the brain was believed to be a continuous network, like a spider’s web, Cajal demonstrated that it was actually composed of individual cells — neurons. In that groundbreaking article, he even envisioned the transmission of nerve impulses, adding arrows to his sketches. For the first time, the movement of sensations and thoughts was depicted.
More than 130 years later, a team led by doctor Sergiu Pasca has used millions of human cells to create a true mini-nervous system in their laboratory, capable of detecting a pain signal and transmitting it right before our eyes. It is, quite literally, human pain served on a plate.
Cajal created modern neuroscience, and Pasca has revolutionized it. In 2015, he used a chemical cocktail to reprogram skin cells, rewinding their development back to an embryonic state and inducing them to become self-organizing neurons in a small cluster. Two years later, he joined a cluster of these cells from the cerebral cortex with another cluster of cells from the deep brain. To his amazement, the two clusters fused, forming functional connections between them. Pasca, a 43-year-old Romanian-American, named these astonishing structures “assembloids.” Three years later, he managed to connect three spheres, containing cells from the cerebral cortex, spinal cord, and muscle. He had recreated the circuitry of voluntary movement.
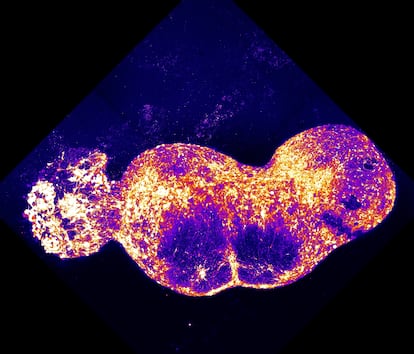
It took Pasca and his team five more years to take the next leap. They modified skin cells from volunteers to turn them into neurons representing the four key regions of the neural pathways that transmit stimuli, such as pain or the sensation of a caress, from the skin to the brain.
This time, four tiny clusters were joined together: spinal ganglion, spinal cord, thalamus, and somatosensory cortex. The result, in Pasca’s words, was “like a tiny sausage.” A two-centimeter-long sausage, composed of four million human cells transmitting a painful stimulus to one another, in his laboratory at Stanford University. His new study was published on Wednesday in Nature.
“Chronic pain is a devastating disorder, affecting almost a quarter of the population. Understanding its biology and finding better treatments is a challenge,” Pasca explains via videoconference. “The scientific community has already studied pain pathways in many ways, but until now, they have been piecemeal: only sensory neurons, only the response of the cerebral cortex… Now we have the advantage of being able to observe all human cells noninvasively, without harming anyone,” he says. Researchers trigger the sensation of pain with substances like capsaicin, found in hot peppers.
An adult human brain is composed of approximately 86 billion neurons. Pasca’s mini-nervous system only has four million and lacks cells from key regions, such as the amygdala, which is linked to the emotion of pain. However, the Stanford doctor believes these assembloids will be very useful in illuminating neurodevelopmental disorders, such as autism spectrum disorders.
“Many people with autism have hypersensitivity to touch, sounds, or visual stimuli. It’s clear that there is something different about how their nervous system processes sensory information,” he argues.

Pasca is hopeful. “Autism, ultimately, is a disorder of social behavior. It involves altered social communication, as well as repetitive movements. Why does it also have a dysfunction in sensory processing? Does that directly contribute to the disorder? Or is it a kind of side effect? We simply don’t know,” he explains. “We can introduce the various genetic mutations associated with autism into assembloids and observe how they disrupt circuits from the outset. It’s a very exciting opportunity to begin to address the biology of this disorder,” he says.
The doctor directs the Stanford Center for Brain Organogenesis, with figures such as U.S. neuroscientist Karl Deisseroth, the father of optogenetics — a revolutionary technique that enables neurons to be turned on or off using bursts of laser light, made possible by the introduction of light-sensitive genes from algae.
In 2022, Pasca, Deisseroth, and their colleagues grafted pellets of human cerebral cortex cells into the brains of rats, resulting in the implanted tissue integrating and influencing the animals’ behavior. A year ago, Pasca’s team used a kind of genetic band-aid to correct an autism-related syndrome in one of these human cerebroids inserted into a rat.
“The new assembloids are not simple structures. They are composed of four parts, measure more than a centimeter, and require about 200 days to make. Although they are challenging to produce, we can imagine that the process will be scalable and thousands upon thousands of assembloids could be created and used to test drugs,” reflects Pasca, whose university has patented the invention. “The best medications we have today for pain are opioids. They are very effective, but they are also highly addictive. To find alternatives, we need to understand the biology behind the circuits,” he explains.
Neurobiologist Félix Viana directs a pain research program at the Institute of Neurosciences in Sant Joan d’Alacant in Spain. “This new work is, technically, very interesting. It’s like four Lego blocks that you can connect, and there’s a signal that goes from the neurons that connect with the outside world to those in the cerebral cortex, but pain circuits are much more complex than that,” says Viana, who did not participate in the study.
For this expert, the mere six months of development of the neurons of the assembloids represents a very early phase, far removed from adult reality. “Furthermore, pain is a subjective sensation,” says Viana. “It’s the subject who has to tell you if they feel it and to what degree. The assembloids will never feel pain. They might be of some interest in preliminary drug analyses, but I’m skeptical that they will produce useful results in the short term.”
Pasca’s team has already taken another significant step, although for now, they have only released preliminary results. The researchers have recreated the cortico-striatal-thalamic-cortical circuit, one of the brain’s key pathways responsible for cognitive, emotional, and motor functions. Once again, the structure consists of four connected spheres, but this time with looped connections, resembling real circuits. The Stanford team has used these more sophisticated structures to study mutations in the ASH1L gene, which is linked to autism spectrum disorders and Tourette syndrome. In the mutated assembloids, the activity is abnormal.
“If you have a pellet of human muscle cells, it doesn’t do much, but if you attach it to a piece of spinal cord, you can control the contraction of that muscle. The brain is more than the sum of its parts,” says Pasca. “When you attach two pellets, new properties emerge. That’s the goal of assembloids: to capture those new properties that arise from the interaction of cells through the nervous system.”
Sign up for our weekly newsletter to get more English-language news coverage from EL PAÍS USA Edition
Tu suscripción se está usando en otro dispositivo
¿Quieres añadir otro usuario a tu suscripción?
Si continúas leyendo en este dispositivo, no se podrá leer en el otro.
FlechaTu suscripción se está usando en otro dispositivo y solo puedes acceder a EL PAÍS desde un dispositivo a la vez.
Si quieres compartir tu cuenta, cambia tu suscripción a la modalidad Premium, así podrás añadir otro usuario. Cada uno accederá con su propia cuenta de email, lo que os permitirá personalizar vuestra experiencia en EL PAÍS.
¿Tienes una suscripción de empresa? Accede aquí para contratar más cuentas.
En el caso de no saber quién está usando tu cuenta, te recomendamos cambiar tu contraseña aquí.
Si decides continuar compartiendo tu cuenta, este mensaje se mostrará en tu dispositivo y en el de la otra persona que está usando tu cuenta de forma indefinida, afectando a tu experiencia de lectura. Puedes consultar aquí los términos y condiciones de la suscripción digital.









































