Study: Under-60s who received first AstraZeneca dose can safely be given Pfizer for second shot
Research from the Carlos III Health Institute has concluded that this method is “highly safe” and more effective against Covid-19 than two injections of the British-Swedish medication

A study in Spain carried out by the Carlos III Health Institute has concluded that the under-60s who have already received one shot of the Oxford-AstraZeneca vaccine can be safely administered the Pfizer-BioNTech medication for their second dose. The recommendation comes after the Spanish Health Ministry suspended the use of AstraZeneca among this age group due to associations with rare cases of blood clots.
A total of 672 volunteers and five hospitals in Madrid, Bilbao and Barcelona took part in the study, officially called CombivacS. The participants had received their first dose of AstraZeneca between eight and 12 weeks previously. They were divided into two groups, with 442 people receiving the Pfizer vaccine for their second dose and 221 as a control group. The latter participants were not given another vaccine, allowing for the two groups to be compared.
More neutralizing antibodies are produced in this study with PfizerAuthors of the Carlos III Health Institute study
According to the Carlos III Health Institute study, the administration of a second dose using the Pfizer vaccine is “highly immunogenic and safe.” The vaccinated group saw its immune response multiply by 120 a week after this second dose, while the control group’s level held steady. When the serum of the patients was mixed in the laboratory with samples of the coronavirus the neutralizing defense response was shown to multiply by seven. In the equivalent trials with a second dose of AstraZeneca, this response was multiplied by three.
“What we have seen in our study is that the increase [using Pfizer for the second shot] is more than seven times. More neutralizing antibodies are produced in this study,” the authors of the CombivacS study said. However, they insisted that this comparison is not an exact one as the methodologies used differed. What’s more, the combination of the Pfizer and AstraZeneca vaccines did not increase side effects.
Speaking at a presentation of the study results on Tuesday, the director of the Carlos III Health Institute, Raquel Yotti, explained that research into the AstraZeneca vaccine “indicates that there is a clear trend of higher incidence [of blood clots] among younger people.” After the Spanish health authorities decided to suspend the use of the medication among the under 60s, she continued, “a need arose to find an alternative for those who had received the first dose.”
Spain had opted to use the vaccine on essential personnel such as teachers and police officers, among others. Since the suspension, this group of some 1.5 million people had been left in limbo about their second shot, and delays to the decision by the health authorities have now seen the first recipients miss the 12-week recommendation by the manufacturer for the administration of the second shot.
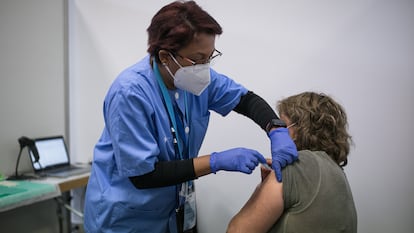
“Some countries decided to offer a second dose of a messenger RNA vaccine,” Yotti continued, in reference to the Pfizer and Moderna vaccines, which use this technology to create an immune response rather than the AstraZeneca vaccine, which uses a weakened common cold virus from chimpanzees that has been genetically modified. “We are staying faithful to the science. The preliminary data has been revised by an independent committee, which has called for them to be made public as quickly as possible. This is what the institute does,” the director continued. “It generates new knowledge.”
The Health Ministry and the regions have been waiting for the results of the Carlos III trial before making a decision on how to proceed with the use of AstraZeneca. The Public Health Commission, which is made up of health authorities from the central and regional government, is expected to set out the next steps Tuesday afternoon now that the results of the testing are in. The lower-than-expected supply of the AstraZeneca vaccine, due to production shortfalls by the manufacturer, is also likely to influence their decision on its use.
As well as essential workers, Spain has opted to use the AstraZeneca vaccine for the 60-69 age group. These recipients are due to receive the two shots, if they haven’t already, and were not affected by the health authorities’ decision to suspend its use in other groups. This age group is also being given the Janssen vaccine, which is a single shot and relies on a similar technology to the AstraZeneca medication.
Political pressure on the central government has been rising in recent weeks over the use of the British-Swedish vaccine. The Andalusia and Madrid regions, both of which are governed by the conservative Popular Party (PP), voiced their complaints last week at a meeting with other regional chiefs and the central Health Ministry about the delay. Officials from both regions called on the government – a center-left coalition of the Socialist Party (PSOE) and junior partner Unidas Podemos – to “find a quick solution to this problem,” according to sources with knowledge of the meeting of the Inter-Territorial Council of the National Health System (CISNS).
English version by Simon Hunter.
Tu suscripción se está usando en otro dispositivo
¿Quieres añadir otro usuario a tu suscripción?
Si continúas leyendo en este dispositivo, no se podrá leer en el otro.
FlechaTu suscripción se está usando en otro dispositivo y solo puedes acceder a EL PAÍS desde un dispositivo a la vez.
Si quieres compartir tu cuenta, cambia tu suscripción a la modalidad Premium, así podrás añadir otro usuario. Cada uno accederá con su propia cuenta de email, lo que os permitirá personalizar vuestra experiencia en EL PAÍS.
¿Tienes una suscripción de empresa? Accede aquí para contratar más cuentas.
En el caso de no saber quién está usando tu cuenta, te recomendamos cambiar tu contraseña aquí.
Si decides continuar compartiendo tu cuenta, este mensaje se mostrará en tu dispositivo y en el de la otra persona que está usando tu cuenta de forma indefinida, afectando a tu experiencia de lectura. Puedes consultar aquí los términos y condiciones de la suscripción digital.








































