Spain opts to use AstraZeneca vaccine for 60- to 69-year-olds
The Public Health Commission has raised the age range after an agreement was reached on the medication by the Health Ministry and the regions. No announcement yet on what will happen for those who have had their first of two shots
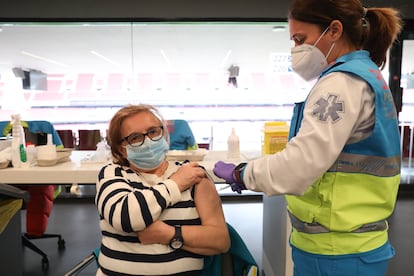
The Public Health Commission of the Spanish Health Ministry decided on Thursday that the Oxford-AstraZeneca Covid-19 vaccine will only be administered to people aged between 60 and 69. The decision raises the upper age limit from 65, which had been set on Wednesday by the Inter-Territorial Council of the National Health System, which brings together central and regional health officials. There are 5.3 million Spaniards in their 70s, of whom one million have already received at least one dose of the Covid-19 vaccines currently available from Pfizer-BioNtech, Moderna and AstraZeneca.
Spain has changed its age limit for the AstraZeneca vaccine repeatedly. First, it set an upper threshold of 55, then raised it to 65 after temporarily suspending its use over a European health scare. Now, the limit is 69.
Up until Wednesday, the AstraZeneca vaccine was only being administered to people under the age of 65 due to concerns over the lack of evidence of its efficiency for the older age group. The Anglo-Swedish medicine was being used to inoculate essential health workers, including teachers and members of law enforcement agencies and emergency services, and to the general population between 60 and 65 years of age.
On Wednesday, however, Spain announced plans to stop using the AstraZeneca vaccine to people who are under 60 years old after the European Medicines Agency (EMA) found a “possible link” between the shots and “very rare cases of unusual blood clots with low blood platelets.”
The Public Health Commission has not yet made a decision on what to do with people under the age of 60 who have already received their first shot of the AstraZeneca vaccine. There are currently three options: use the AstraZeneca vaccine for the second shot, given that all blood clot cases so far have happened after the first injection; use a different vaccine for the second dose, the effectiveness of which will be revealed in trials set to be published in the coming weeks; or not administer the second dose, as the first is considered to be 70% effective against Covid-19.
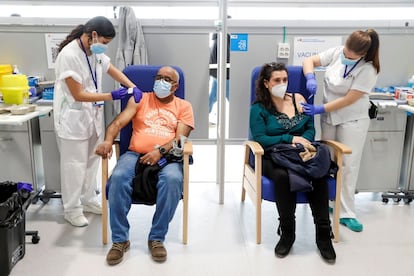
An argument in favor of delaying a decision on what approach to take is the fact that up to 12 weeks can pass between the first and second shot of AstraZeneca, meaning there is time for new data to be released. A total of 2,310,933 people in Spain have received a first dose of the vaccine, while just 119 people have already been given the two shots that offer full protection.
The decision to restrict the AstraZeneca vaccine to the 60 to 69 age group has left essential workers in limbo. In Cantabria, a spokesperson from the health department said that the region has had to cancel most of the vaccinations scheduled for 917 police officers and teachers due to the change in criteria. In Galicia, 1,403 essential workers under the age of 60 were set to receive the AstraZeneca vaccine on Thursday – appointments that had to be canceled. In Madrid – the only region to vote against the change in criteria – a spokesperson from the health department said 600 appointments for essential workers, including teachers, firefighters and other emergency workers, were also suspended.
Vaccination drive on track
Spanish Health Minister Carolina Darias said on Thursday that despite the new age restrictions on the AstraZeneca vaccine Spain was still on track to vaccinate 33 million people, or 70% of the adult population, by September.
But health experts argue that reaching this goal will depend on the supply of other vaccines, particularly the Janssen vaccine, which only requires one dose for full protection.
“The AstraZeneca issue does not help to achieve this goal [of vaccinating 33 million people], but it may not compromise it if other vaccines can supplement its planned vaccinations,” said Alberto Infante, professor of the National Health School at the Carlos III Health Institute.
Experts also worry that the constant strategy changes will further erode the public’s confidence in vaccines. “It has a big impact on the public, they are afraid and this makes the act of vaccinating more difficult,” explained epidemiologist Jesús Molina Cabrillana. “You have to explain to people what you are injecting and many people have doubts, meaning you have to invest more time in calming that anxiety.”
With reporting by Juan Navarro, Juan José Mateo, Cristina Huete, Eva Saiz, María Fabra, Mikel Ormazabal and Diego Estébanez.
English version by Melissa Kitson.
Tu suscripción se está usando en otro dispositivo
¿Quieres añadir otro usuario a tu suscripción?
Si continúas leyendo en este dispositivo, no se podrá leer en el otro.
FlechaTu suscripción se está usando en otro dispositivo y solo puedes acceder a EL PAÍS desde un dispositivo a la vez.
Si quieres compartir tu cuenta, cambia tu suscripción a la modalidad Premium, así podrás añadir otro usuario. Cada uno accederá con su propia cuenta de email, lo que os permitirá personalizar vuestra experiencia en EL PAÍS.
¿Tienes una suscripción de empresa? Accede aquí para contratar más cuentas.
En el caso de no saber quién está usando tu cuenta, te recomendamos cambiar tu contraseña aquí.
Si decides continuar compartiendo tu cuenta, este mensaje se mostrará en tu dispositivo y en el de la otra persona que está usando tu cuenta de forma indefinida, afectando a tu experiencia de lectura. Puedes consultar aquí los términos y condiciones de la suscripción digital.








































