Genetically modified pig liver transplanted into a living person for the first time
The auxiliary graft functioned well for the first month, but had to be removed due to complications. The 71-year-old patient, who suffered from cirrhosis and liver cancer, died four months later
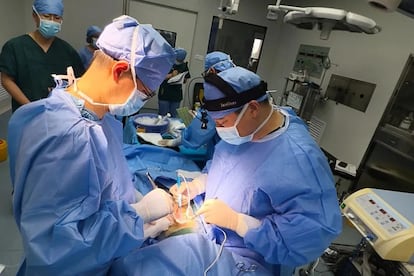
The path toward using genetically modified animal organs for human transplants seems increasingly clear. But it’s a road with significant obstacles, as demonstrated by a case that came to light on Thursday: the first living person to receive a genetically modified pig liver transplant. This 71-year-old patient, who had cirrhosis and liver cancer, was able to use the organ for over a month, during which time it functioned properly.
But on day 38, complications arose that forced the process to be reversed, and the man ultimately died four months later, on day 171 after the transplant. The case shows that, although possible, it is difficult going in a field where the need for viable organs is acute.
“This case demonstrates that a genetically modified pig liver can function in humans for an extended period,” says the study’s principal investigator, Beicheng Sun, of First Affiliated Hospital of Anhui Medical University, Hefei, China. “This is a crucial step that demonstrates both the potential and the remaining obstacles, especially regarding coagulation problems and immune complications that must be resolved,” the specialist acknowledged in a statement published by the Journal of Hepatology, which published the achievement.
In China, where this patient was from, hundreds of thousands of people suffer liver failure each year, but in 2022, only about 6,000 received a transplant. In Europe, last year there were more than 22,000 patients on a waiting list for a liver transplant, and of those, only half received the desired transplant, while more than 2,300 patients died on the waiting list. According to the World Health Organization, thousands of patients die each year waiting for an organ transplant due to a shortage of human donors.
In recent years, pig hearts, kidneys and lungs have also been successfully transplanted into human patients, in a field that proves to be a hopeful avenue for millions of people. But some patients were brain-dead, and others died shortly after receiving the organ, so progress remains modest.

This latest procedure was performed on a 71-year-old man with cirrhosis due to hepatitis B and carcinoma, who was not a candidate for a human liver transplant. Surgeons implanted an auxiliary graft from a pig with 10 genetic modifications, including the introduction of human genes to improve immune compatibility and reduce the risk of clotting.
The operation was performed on May 17, 2024, and a week later everything was going well, according to the university: “The patient was able to walk freely, no hyperacute or acute rejection reactions were found, the coagulation system was not affected, and liver function had returned to normal.”
But after a month of producing bile without problems, complications emerged necessitating the removal of the graft. The patient subsequently suffered several episodes of gastrointestinal bleeding and died.
This technique, known as xenotransplantation (from a species other than humans), had already been tested with pig livers in two people, one in China and one in the United States, but in a brain-dead state. The Chinese transplant was performed in March 2024 by a team led by surgeon Lin Wang. “It worked very well in the human body,” said the doctor. Their work was published in May in Nature, a much more influential showcase than The Journal of Hepatology.
The difference is that this time, an auxiliary transplant was performed without removing the patient’s organ entirely. The study published in Nature was the first proof of concept, as a pig liver was successfully ignited in the recipient despite their condition, and continued to function for 10 days (until the family asked for the experiment to be terminated).
Dr. Beicheng Sun responds with a concise “yes, I agree” when asked if his patient’s outcome shows that these scientific steps are more complicated than they seem. EL PAÍS received the same response to the question of whether China is ahead of the United States in this scientific race for a viable and permanent xenotransplant. Sun received significant attention from the Chinese media when the transplant was announced (they even published a photo of the patient), but he did not publish details of his scientific work until this Thursday. Two months after the operation, China sanctioned Sun for unethical conduct in accessing public funds for his research.
It’s not an open door yet
“From a scientific perspective, this case demonstrates that a genetically modified pig liver can be grafted, function, and provide synthetic support in a human recipient, at least for several weeks,” states an editorial accompanying the study, signed by a group of specialists. The four authors, led by German author Heiner Wedemeyer, conclude: “This study should be read both as a cause for cautious optimism and as a reminder of how much progress remains to be made.” They caution, above all, that “this operation does not yet open the door to widespread clinical use of pig livers, but it does establish proof of concept that such grafts can work in humans.”
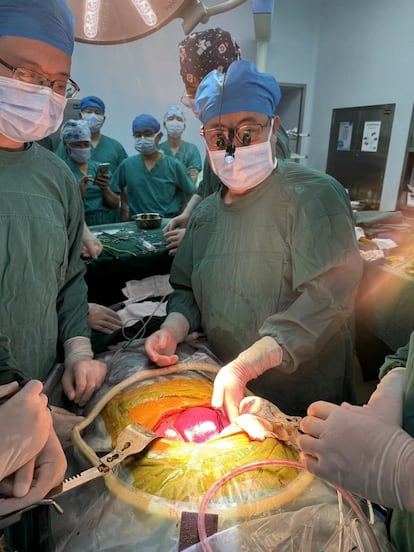
Beatriz Domínguez-Gil, director of the Spanish National Transplant Organization (ONT), emphasizes that this xenotransplant is “the first one performed for therapeutic purposes.” “It demonstrates that the liver is capable of functioning and supporting the patient for 38 days without evidence of rejection. However, the patient ends up developing thrombotic microangiopathy related to the xenotransplant, which requires graft resection,” Domínguez-Gil told the specialized platform SMC Spain.
“We can say that this is a new step in the advancement of xenotransplantation therapy, which continues to progress in its clinical development, but it also demonstrates the significant obstacles that still need to be overcome, such as the serious complications observed in this patient,” Domínguez-Gil adds. “However, these cases allow us to glimpse a future in which xenotransplantation will be a clinical reality as a bridge therapy (particularly in the case of the liver) or as a destination therapy,” the specialist celebrates.
In statements to the same website, both Rafael Matesanz, founder of the ONT, and Iván Fernández Vega, scientific director of the Biobank of the Principality of Asturias, point out the strengths and limitations of the breakthrough. “Within the race established in recent years by researchers in the United States and China to take the lead in the field of xenotransplantation, the team at Anhui University Hospital has taken a very important step with this study,” notes Matesanz. He adds: “The experience is highly relevant. As with other xenotransplantation experiments, there are more questions than answers, but a giant step has been taken with a possible immediate practical application as a bridge organ, something that until now had not credibly occurred with other transplanted porcine organs.” For Fernández Vega, “it marks a historic milestone” and the study “is very well documented, with detailed clinical, immunological, and histological follow-up, which gives it great scientific value and reference for the field.” “Xenotransplantation was not intended as a curative cancer treatment, but rather as a supportive strategy to prevent liver failure after tumor removal, since the remaining liver was insufficient,” explains this researcher from the University of Oviedo.
On the other hand, Matesanz warns that “long-term survival is still a long way off, as with other porcine organs transplanted so far.” And Fernández Vega regrets that the study does not describe the results of an autopsy, “to confirm the absence of tumor recurrence, evaluate the condition of the native liver, better understand hemorrhagic complications, and study the systemic immune response. Important questions also remain regarding safety against porcine viruses, duration of function, and ethical and social acceptance,” he concludes.
Sign up for our weekly newsletter to get more English-language news coverage from EL PAÍS USA Edition
Tu suscripción se está usando en otro dispositivo
¿Quieres añadir otro usuario a tu suscripción?
Si continúas leyendo en este dispositivo, no se podrá leer en el otro.
FlechaTu suscripción se está usando en otro dispositivo y solo puedes acceder a EL PAÍS desde un dispositivo a la vez.
Si quieres compartir tu cuenta, cambia tu suscripción a la modalidad Premium, así podrás añadir otro usuario. Cada uno accederá con su propia cuenta de email, lo que os permitirá personalizar vuestra experiencia en EL PAÍS.
¿Tienes una suscripción de empresa? Accede aquí para contratar más cuentas.
En el caso de no saber quién está usando tu cuenta, te recomendamos cambiar tu contraseña aquí.
Si decides continuar compartiendo tu cuenta, este mensaje se mostrará en tu dispositivo y en el de la otra persona que está usando tu cuenta de forma indefinida, afectando a tu experiencia de lectura. Puedes consultar aquí los términos y condiciones de la suscripción digital.









































