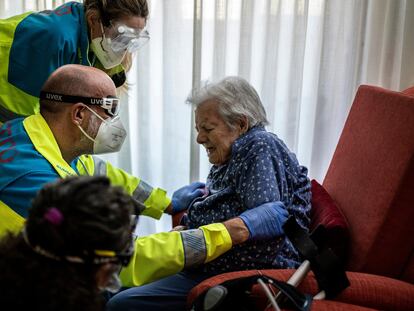
The differences between the Pfizer, Moderna and Oxford coronavirus vaccines: what we know so far
Price and storage temperatures are among the various factors that set each of the three most promising candidates apart
In a little more than 10 months, researchers have managed to develop three vaccine candidates that are reported to be highly effective in preventing the coronavirus. But each comes with its own distinct advantages and disadvantages. Here is an overview of the main differences.
Price
Most people in the world will need to be vaccinated against the coronavirus, so the price of the injection is an essential factor in making the drug accessible to all. The price difference between the three vaccines is significant: the one developed by Oxford University and the biopharmaceutical company AstraZeneca will cost around €3 per dose; the one developed by the US multinational Pfizer and the German biotech company BioNTech will cost more than €15 per dose; and the one developed by the US company Moderna and the US National Institutes of Health will cost €21 per dose, seven times more than the Oxford vaccine.
How they work
Storage temperature
Getting coronavirus vaccines to every corner of the world will be an unprecedented logistical challenge. One of the decisive factors will be the temperature at which each product must be stored. Oxford University has stated that its vaccine can be kept in a refrigerator, at temperatures of between 2 degrees Celsius and 8°C, and can therefore be distributed using the existing channels for other vaccines. Pfizer’s vaccine, meanwhile, requires ultra-cold temperatures of about -70°C, a problem the company will try to solve by using dry ice containers that can keep the vaccine at the right temperature for 15 days. The storage of Moderna’s product lies somewhere between the two. Its vaccine remains effective for at least six months at -20°C and can be kept for 30 days in a refrigerator, at temperatures of between 2°C and 8°C.
Effectiveness
The three teams have declared that each vaccine is between 90% and 95% effective, with some grey areas. Oxford University and AstraZeneca announced on November 23 that their vaccine was up to 90% effective, based on a trial involving 2,700 volunteers who initially received just half a dose and got a second injection with the full dose a month later. Curiously, two full doses reduced the vaccine’s effectiveness to 62%, according to the results from a larger trial involving 8,900 participants. The phenomenon is under examination and it remains to be seen if the preliminary 90% rate of effectiveness is maintained in the final results of its clinical trial, which has already recruited some 24,000 volunteers in the United Kingdom, Brazil and South Africa.
Pfizer and BioNTech were the first to announce their vaccine’s effectiveness, which stands at 95%, a figure that has complete statistical validity following a trial involving around 44,000 participants, 170 of whom had the virus. Only eight of these positive cases received the real vaccine as opposed to the placebo. Pfizer also clarified that the vaccine’s 95% effectiveness remained consistent across all age groups, genders and races.
Meanwhile, the US company Moderna and the National Institutes of Health, have announced their vaccine to be 94% effective, based on the final results of a trial involving 30,000 people in the US.
Vaccines bought by the EU
All three vaccines require two doses. In the case of the Pfizer vaccine, these doses would be 21 days apart; in the case of the Oxford and Moderna vaccines, the period between doses is one month. The European Commission has reached a deal with the biopharmaceutical company AstraZeneca to buy 300 million doses of the Oxford vaccine, with an option to buy 100 million more. The European authorities have also made a deal with Pfizer-BioNTech for 200 million doses, with an option for an additional 300 million. And the European Commission continues negotiating with Moderna, with which it has a preliminary deal for at least 80 million doses, with an option for an added 160 million. On Monday, Moderna announced that it planned to request conditional marketing authorization in Europe.
The EU has around 450 million inhabitants and has already made deals to buy more than 1.3 billion doses of vaccine candidates, including those in the pipeline from Germany’s Curevac, the US’ Johnson & Johnson and the alliance formed by France’s Sanofi and Britain’s GSK.
English version by Heather Galloway.