Spain authorizes Phase 3 trial of Johnson & Johnson coronavirus vaccine
Nine Spanish hospitals will participate in a test that seeks to analyze the efficacy and safety of the drug on 30,000 volunteers from nine countries
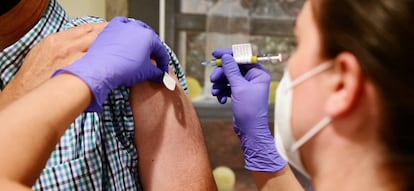

The Spanish Health Ministry on Wednesday announced that the drug approval agency AEMPS has authorized a Phase 3 clinical trial of the Covid-19 vaccine developed by Janssen, a subsidiary of Johnson & Johnson. This is the first late-stage trial that has been authorized in Spain.
In a press release, the ministry said that the testing will follow a “double-blind” design in which two doses of the real vaccine and placebos will be administered to 30,000 volunteers to be recruited in Spain, Belgium, Colombia, France, Germany, the Philippines, South Africa, United Kingdom and the United States. Nine Spanish hospitals will participate and start searching for volunteers “as soon as possible,” said the release.
The European Commission has signed a preliminary agreement to buy 200 million doses of the Janssen vaccine
The Janssen vaccine is based on a common cold virus called adenovirus, which has been modified with genetic information from the coronavirus in a bid to get the body to create specific defenses without posing any health risk.
Only volunteers without pre-existing medical conditions associated with a risk of developing serious Covid-19 symptoms will be admitted in the initial stage of testing. An independent committee will assess the safety of this stage, which will be followed by a second stage of testing on individuals with comorbidities that could pose a risk of developing Covid-19.
All experimental vaccines must go through three phases of human trials: one with a few dozen healthy individuals to rule out serious side effects, another one with hundreds of people to confirm the results and adjust the dosage, and a third with tens of thousands of participants to prove the drug’s safety and efficacy.
The European Commission has signed a preliminary agreement to buy 200 million doses of the Janssen vaccine, as well as deals with other pharmaceutical giants such as Pfizer. There are at least 234 vaccine projects in development across the world.
In the space of just one week, three competitors have reported high efficacy in their coronavirus vaccines: Pfizer-BioNTech (90%), Russia with its Sputnik V vaccine (92%) and Moderna (over 94%). But this information has been provided by the sources themselves, not through any independent channels, which would require the publication of the clinical trials in a well-respected medical journal.
English version by Susana Urra.
Tu suscripción se está usando en otro dispositivo
¿Quieres añadir otro usuario a tu suscripción?
Si continúas leyendo en este dispositivo, no se podrá leer en el otro.
FlechaTu suscripción se está usando en otro dispositivo y solo puedes acceder a EL PAÍS desde un dispositivo a la vez.
Si quieres compartir tu cuenta, cambia tu suscripción a la modalidad Premium, así podrás añadir otro usuario. Cada uno accederá con su propia cuenta de email, lo que os permitirá personalizar vuestra experiencia en EL PAÍS.
¿Tienes una suscripción de empresa? Accede aquí para contratar más cuentas.
En el caso de no saber quién está usando tu cuenta, te recomendamos cambiar tu contraseña aquí.
Si decides continuar compartiendo tu cuenta, este mensaje se mostrará en tu dispositivo y en el de la otra persona que está usando tu cuenta de forma indefinida, afectando a tu experiencia de lectura. Puedes consultar aquí los términos y condiciones de la suscripción digital.








































