Anti-aging science: In search of eternal youth
A new technique for regenerating cells is not ready for humans, but has potential for extending the quality of life
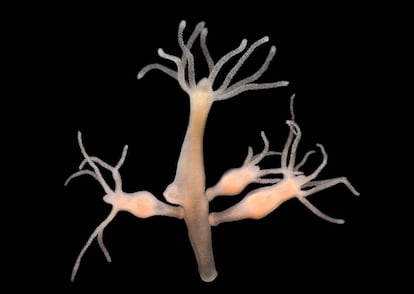
There is an animal that is almost immortal – it never ages. Less than half an inch (1 cm) in size, the hydra is a tiny freshwater invertebrate blessed with eternal youth. The discovery happened in 1998 when Daniel Martínez, a professor of molecular biology at Pomona University (California), was conducting experiments to prove exactly the opposite. “I decided to experiment with the hydra to prove that it ages because all animals age. But four years later, I still didn’t see any mortality. That’s extraordinary for a little creature like the hydra, because the lifespans of tiny things are usually measured in weeks, not years,” he said. The central tenet that all forms of life eventually die was shaken to its core by the tiny hydra. The scientific community was astounded. How is it possible? What’s so special about the hydra? And most importantly, could this be true of other animals, perhaps humans?
The secret to the hydra’s eternal youth is found in its body, says Martínez, which is made entirely of stem cells. The hydra is constantly regenerating itself, something that is impossible for humans. “We aren’t able to have bodies made entirely of stem cells because we need organs,” said Martínez. “Since we are so large, we can’t absorb food through our skin. We need systems to ingest food and carry nutrients throughout the body. Human organs need differentiated cells and that causes them to lose their capacity to reproduce. If they regain this capacity, cancers can grow. So, once the cells decide they’re going to differentiate, our bodies have control mechanisms to make sure they don’t reproduce. If those don’t work, then we have problems.” In short, the complexity of our bodies is what ultimately kills us.
An Argentine who has lived for many years in California, Martínez completely rejects the notion that eternal youth is possible for humans. But he acknowledges that the hydra could help scientists decipher the molecular mechanisms that cause aging in humans. This question has been challenging the scientific community for decades, but recent discoveries have reinvigorated anti-aging research.
When Shinya Yamanaka demonstrated in 2006 that adult cells could be “rewound” back to embryonic stages, it revolutionized the science of aging. While this mechanism is nowhere near ready for human application, the scientists have redoubled efforts to slow aging processes that are associated with diseases like cancer and Alzheimer’s. Scientists are using cellular reprogramming and other techniques to find ways of neutralizing or reversing the processes of cellular decrepitude. But they are careful to say that we will never be immortal. Death is inevitable and accidents happen. The goal is to grow old in good health.
For many years now, people have been living longer and longer. Improved health care, antibiotics and vaccines have reduced mortality and extended life expectancy around the world to an average of 73 years (it was 53 years in 1960). As life expectancy continues to rise, scientists debate whether there is a limit. A 1996 study estimated the maximum lifespan at 120 years, while a 2016 study put it at about 125 years. The longest-living person so far was Jeanne Calment, a French woman who died in 1997 at 122 years and five months.
Pura Muñoz-Cánoves, a university professor and researcher with Spain’s National Center for Cardiovascular Research, says that the average lifespan will continue to rise, but there is a limit. “At the rate we’re going and with all research being conducted, aging can be slowed down. But we are still mortal and someday we’re going to die. The idea is that we will have better quality of life and good health in our last years,” said Muñoz-Cánoves. Although the average lifespan has risen, she says, no one has topped Jeanne Calment’s 122 years. “Instead of trying to surpass the maximum, we want people who are now 80 or 90 to reach the age of 100 in good health,” she said. “Today’s newborns can expect to live 100 years.”
Cell damage
Simply speaking, aging is the result of accumulated cell damage and the loss of normal cell function, and scientists want to understand the underlying molecular mechanisms of this process. “Why does damage accumulate [with age]? Because there is more opportunity for damage from external attacks like sun exposure and lifestyle habits. Also, our cells lose the capacity for self-repair and cleansing,” said Muñoz-Cánoves.
In a 2001 paper published in Cell, María Blasco, director of Spain’s National Cancer Research Center, identified nine molecular hallmarks of aging. Blasco and her team analyzed hallmarks like genomic instability (defects that accumulate in genes over time) and loss of proteostasis, which involves failures in the mechanisms for eliminating defective proteins that can cause disease.
Aging has many causes, says Blasco, including telomere shortening, a principal research focus for her. Telomeres are sections of DNA found at the ends of each of our chromosomes that shorten with each round of cell division. “We know that aging is triggered when telomeres are too short. If you lengthen the telomeres in mice, they live longer.”
Aging also results from failures in the nutrient sensing system, a physical mechanism that is activated when food is present or absent. Similarly, aging results from epigenetic alterations affecting the molecules that attach and interact with DNA. A study published in the Journal of the American Medical Association (JAMA) identified causes of aging such as changes in stem and progenitor cells that reduce their capacity to repair or replace tissues, and chronic but sterile low-grade inflammation not caused by any known pathogens. Muñoz-Cánoves also points to a reduced capacity for autophagy (the body’s cellular recycling system). “It’s as if age prevents the body’s vacuum cleaner from working efficiently to eliminate molecules that aren’t functioning well in the cells, so this dirt just keeps building up.”
Cellular senescence is yet another mechanism that leads to aging. Cells have a kind of programmed shutdown system to protect themselves. When they encounter irreparable internal damage, cells stop dividing (senescence), but they don’t die. In older tissues, senescent cells accumulate and can release compounds “that damage the surrounding environment and produce inflammation,” said Muñoz-Cánoves.
Researchers are hard at work studying all the mechanisms that ultimately lead to disease. But no silver bullet has been discovered, says Muñoz-Cánoves, only a few palliative measures like the senolytic drugs that selectively clear senescent cells. “Experiments with mice show that using senolytics, which eliminate senescent cells and increase the capacity for autophagy, produces cleaner cells,” said Muñoz-Cánoves.
To counteract telomere shortening, Blasco advocates the use of telomerase activators. Telomerase is an enzyme that enables telomere DNA to regenerate, thereby slowing down the aging process. “Telomerase repairs telomeres and avoids the shortening that occurs. Very short telomeres cause significant damage because they induce cells to either stop dividing or to kill themselves off. In both cases, they lose their regenerative capacity,” write Blasco and co-author Mónica Salomone in Morir joven, a los 140 (or Dying Young at 140).
The search for anti-aging pharmaceuticals has led to tests of drugs like metformin, a well-known Type-2 diabetes medication. The idea is to try and stave off ailments associated with old age, such as cancer and dementia. Leading this effort is Nir Barzilai, director of the Institute for Aging Research at Albert Einstein College of Medicine in New York. In 2016, Barzilai told The New York Times, “Our goal is to… extend health span. The best we can expect from metformin is two or three additional years of healthy aging. But the next generation of drugs will be much more potent.” In 2002, the US National Institute on Aging launched the Intervention Testing Program, an effort to find potential effective anti-aging agents. So far, nine compounds have been identified that could play a role in extending life expectancy.
Cellular reprogramming
Shinya Yamanaka won the Nobel Prize for Medicine in 2012 for cellular reprogramming, a therapy that is currently in vogue. The technique has already been tested on human in-vitro cells and mice cells. Juan Carlos Izpisua, a Spanish scientist at the Salk Institute for Biological Studies (San Diego, California), demonstrated that he could prolong the lives of mice with progeria (premature aging) by combining “Yamanaka factors” (a group of protein transcription factors that play a vital role in the creation of stem cells that have the ability to become any cell in the body).
Most aging experts remain cautious. “The tests on mice and human cultured cells are encouraging. But it’s very difficult to set target dates. Tissue reprogramming in humans is not yet possible, but we do have a solid proof-of-concept,” said Muñoz-Cánoves. Blasco is even more cautious and characterizes human cell reprogramming as a “futuristic” technique. “Using reprogramming, you can indeed lengthen the telomeres, but you also change the identity of the cell and that’s dangerous. Reprogramming takes away the cell’s personality and makes it go backward. And if you no longer know the identity of the cell, you lose control of what’s happening. I think it’s a long way off,” she said.
Muñoz-Cánoves readily acknowledges the issues with cell reprogramming, but is still encouraged by the latest results. “Izpisua’s recent study showed how cells can be reprogrammed by manipulating the [Yamanaka] factors without losing their identity. There is no need to go back to the embryonic stage. It just involves applying a few, controlled doses that slightly reset the cells.” Izpisua tested the effects of long-term partial reprogramming in healthy mice, and found that it leads to rejuvenating effects in different tissues, such as the kidney and skin, and at the organismal level.
In practice, says Muñoz-Cánoves, it may not be necessary to reprogram every cell. “Comorbidities appear with old age because dysfunction in an important organ leads to other problems. Perhaps by rejuvenating a dysfunctional organ we could prevent the cascade of diseases and avoid touching the rest of the organism.”
In any case, the field of cellular rejuvenation has attracted a lot of interest. Muñoz-Cánoves will be leaving her position soon at Pompeu Farba University (Barcelona, Spain) to join Altos Labs, a new US-based life science company funded by billionaires like Jeff Bezos. Altos Labs is focused on developing therapies that can halt or reverse the human aging process, and has also enlisted Juan Carlos Izpisua and Shinya Yamanaka, who will serve as a scientific advisor.
Staying healthy in old age
The elixir of life, fountain of youth, rejuvenation potion. Call it what you will, experts say it doesn’t exist. Salvador Aznar Benitah, a group leader at the Institute for Research in Biomedicine in Barcelona, said, “While Yamanaka demonstrated that it’s not impossible to return to an embryonic state… and you can reprogram aged tissue to rejuvenate, but the animal always dies in the end. We should not think about immortality.” Muñoz-Cánoves agrees: “Youth is not eternal, but there will be healthy aging, without all the chronic ailments.”
Immortality and eternal youth is still a fantasy, and Daniel Martínez thinks it’s better this way. “I don’t think we will ever stop growing old. What’s more, if that does become possible someday, we will have to decide who wants to age and who doesn’t. Personally, I would not be interested in staying forever young. Life would seem too boring if I had all the time in the world. What if Beethoven knew that he would live for 500 years… would he have been able to produce all that spectacular music?”
Tu suscripción se está usando en otro dispositivo
¿Quieres añadir otro usuario a tu suscripción?
Si continúas leyendo en este dispositivo, no se podrá leer en el otro.
FlechaTu suscripción se está usando en otro dispositivo y solo puedes acceder a EL PAÍS desde un dispositivo a la vez.
Si quieres compartir tu cuenta, cambia tu suscripción a la modalidad Premium, así podrás añadir otro usuario. Cada uno accederá con su propia cuenta de email, lo que os permitirá personalizar vuestra experiencia en EL PAÍS.
¿Tienes una suscripción de empresa? Accede aquí para contratar más cuentas.
En el caso de no saber quién está usando tu cuenta, te recomendamos cambiar tu contraseña aquí.
Si decides continuar compartiendo tu cuenta, este mensaje se mostrará en tu dispositivo y en el de la otra persona que está usando tu cuenta de forma indefinida, afectando a tu experiencia de lectura. Puedes consultar aquí los términos y condiciones de la suscripción digital.









































