This is how the brain behaves during a psychedelic trip
So-called ‘magic mushrooms’ contain psilocybin, a hallucinogenic substance that has therapeutic potential in the field of psychiatry. It alters a brain network involved in introspective thinking, leading us to daydream and remember
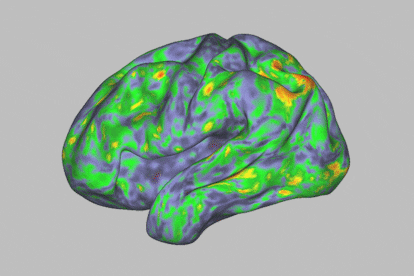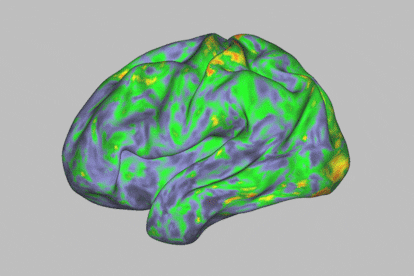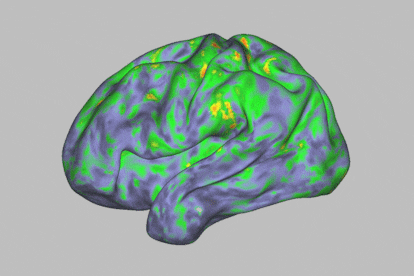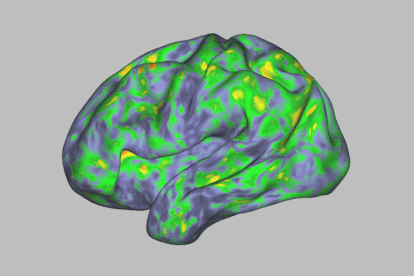
At first glance, during a psychedelic trip with psilocybin, one enters a kind of trance. This substance — which is present in certain hallucinogenic (or “magic”) mushrooms — leads to an altered state of consciousness. It makes human beings much more sensitive to their environment, while offering us the ability to experience visual disturbances, synesthesia phenomena and even mystical experiences. The self dissolves and transcends the body, while spatiotemporal dimensions become distorted. All this happens in a span of about four to six hours. The timeframe also opens the door to exploiting this journey for therapeutic purposes: the psychoactive substance is thought to have potential against particular psychiatric disorders, such as resistant depression or addictions.
Amidst the renaissance in psychedelic medicine, science has turned to studying what’s behind these unusual mental experiences that hallucinogens lead to. Research published in the scientific journal Nature has taken a leap forward in understanding how the brain works in these circumstances, by describing the brain changes that occur in a handful of healthy people during these trances. The study sheds light on a neurobiological explanation for these so-called “trips,” revealing that psilocybin alters a brain network involved in introspective thinking, such as daydreaming and remembering.
Researchers at Washington University in Saint Louis, Missouri monitored the brain activity of seven healthy individuals who were given high doses of either psilocybin or methylphenidate, a stimulant intended to treat attention deficit disorder and hyperactivity. Experts consulted by EL PAÍS say that the reason for comparing these two substances was to avoid unintentionally influencing the participants in the trial: “In clinical trials, with this type of substance, the person already has expectations that something is going to happen to them. And, if they don’t notice anything, the brain also reacts. Stimulants are given — such as methylphenidate — because they also produce a [chemical] activation very similar to that of psilocybin, although it doesn’t have psychedelic effects. This way, suggestion is avoided,” says Víctor Pérez, head of Psychiatry at Hospital del Mar in Barcelona. The doctor didn’t participate in this particular study, but he knows this compound well and has collaborated in other research projects involving psilocybin to treat depression.
To connect the subjective experiences of these trances with a neurobiological explanation, the researchers subjected all participants to neuroimaging tests before, during and after taking them. “Today, we know a lot about the psychological and molecular or cellular effects of psilocybin. But we don’t know as much about what happens at the level that connects the two: the functional brain networks,” Joshua S. Sieger — the study’s first author — explained in a statement.
The human brain is made up of 100 billion neurons connected to each other and arranged in intricate networks that synchronize their activity to construct thoughts, behaviors and emotions. The scientists’ analysis revealed that psilocybin massively altered functional connectivity (communication between different brain areas) in the cortex and subcortex of the brain, causing much more acute changes than those generated by methylphenidate.

Specifically, the most profound changes were seen in the default mode network, a set of brain regions associated with automatisms: the activities that occur when the mind is at rest. “It’s an area related to the substrate of our identity, with our ego,” says Óscar Soto, a psychiatrist and president of the Spanish Society of Psychedelic Medicine. This part of the brain is often altered when a person is afflicted by certain psychiatric disorders.
The study revealed that, during the psychedelic trip, the usual patterns of neuronal communication throughout the brain are altered in a generalized way. But the most acute changes are driven by a kind of desynchronization in the default mode network, between neurons that normally activate each other. “After a person takes the psychedelic compound psilocybin, some of their brain networks dissolve — especially the one involved in the perception of self, space and time. Changes to the connections to this network can last for weeks,” summarizes Petros P. Peridis, a professor in the Department of Psychiatry at New York University, in an accompanying analysis in Nature.
Additionally, changes in neuronal communication throughout the brain were correlated with the intensity of the subjective psychedelic experience. “Psilocybin is capable of inducing changes in the synchrony of the default network. This desynchronization is associated with the psychedelic effect, because when you don’t have these effects, no changes are seen in the network,” Dr. Pérez explains.
Pérez argues that, while this study was done with healthy participants — and while the brain networks of those suffering from mental health disorders are differently affected — the data gathered by the study offers a model “that most closely approximates what happens in the neural network of a patient.” The authors see changes in a person’s default neural network: it’s activated when you give it psilocybin and it’s activated differently when it receives methylphenidate. “This is related to the psychedelic effect that the patient has, such as hallucinations, changes in how one feels, mystical ideas, or the dissociative effect, in which the self merges with the environment. And then, a few weeks later, [researchers observe that] changes persist in the hippocampus,” he notes.
In the days following the administration of psilocybin, most brain networks normalized. However, the changes caused by the psychoactive substance persisted in the connections between the default network and the anterior hippocampus, the area that stores memories associated with emotions. It’s one of the few parts of the brain where new neurons are formed.
“There’s a massive effect initially, and when it’s gone, a pinpoint effect remains,” says Nico U. F. Dosenbach, a professor of neurology and co-author of the study, in a statement. “That’s exactly what you’d want to see for a potential medicine. You wouldn’t want people’s brain networks to be obliterated for days, but you also wouldn’t want everything to snap back to the way it was immediately. You want an effect that lasts long enough to make a difference.”
Persistent changes
The researchers found that, in the hippocampus, the changes were maintained and there was generation of new neurons. In the eyes of Dr. Pérez, this is a key finding: “There’s a centralized point of change in the hippocampus, which is an area where — in depressed patients — neurogenesis atrophies and disappears. In fact, drugs that treat depression activate this formation of neurons in the hippocampus and prevent it from atrophying. It’s very telling to [observe] that psilocybin is related to the neurogenesis [detected in the study].”
For psychiatrist Óscar Soto — who also didn’t participate in this research — the study “reinforces the theoretical paradigm” on which the researchers based the potential of this psychedelic. “That the changes persist in the long-term is very relevant, because with specific [drug regimens], we achieve persistent changes.”
Along the same lines, Petridis thinks that the findings have “significant clinical implications, because [they suggest] that psilocybin could make the brain more malleable, which could be beneficial for people who suffer from rigid maladaptive patterns of thought and behavior.” He goes into more depth in his reflection, noting that it’s conceivable that this malleability could be used to help patients suffering from addiction as they “reframe their relationship with substances in the days and weeks following a dose of psilocybin.”
When it comes to resistant depression, there are already studies that confirm the benefits of psychedelic treatments. Today, Australia allows psychedelic drugs to be administered to patients in this context. And Soto also points out that its use is already being explored to treat those living with addictions, anorexia and obsessive-compulsive disorders.
Translated by Avik Jain Chatlani.
Sign up for our weekly newsletter to get more English-language news coverage from EL PAÍS USA Edition
Tu suscripción se está usando en otro dispositivo
¿Quieres añadir otro usuario a tu suscripción?
Si continúas leyendo en este dispositivo, no se podrá leer en el otro.
FlechaTu suscripción se está usando en otro dispositivo y solo puedes acceder a EL PAÍS desde un dispositivo a la vez.
Si quieres compartir tu cuenta, cambia tu suscripción a la modalidad Premium, así podrás añadir otro usuario. Cada uno accederá con su propia cuenta de email, lo que os permitirá personalizar vuestra experiencia en EL PAÍS.
¿Tienes una suscripción de empresa? Accede aquí para contratar más cuentas.
En el caso de no saber quién está usando tu cuenta, te recomendamos cambiar tu contraseña aquí.
Si decides continuar compartiendo tu cuenta, este mensaje se mostrará en tu dispositivo y en el de la otra persona que está usando tu cuenta de forma indefinida, afectando a tu experiencia de lectura. Puedes consultar aquí los términos y condiciones de la suscripción digital.









































