Aissam, the boy who escaped from a world of silence thanks to gene therapy: ‘I’m hearing the phone!’
The 12-year-old boy is the first person in Europe and the United States with congenital deafness that was able to hear thanks to gene therapy: he’s recovered his hearing, although he will probably never fully develop the ability to speak
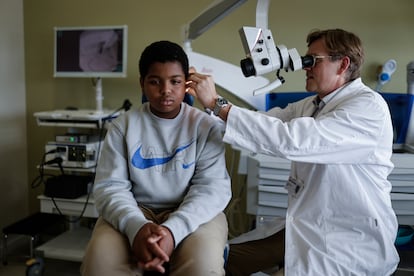
Aissam Dam lived in a bubble of absolute silence that had lasted the young boy’s entire life. He spent 11 years in a noise-proof penumbra. Not a word, not a shout, not a single voice penetrated the walls of the rare congenital deafness that had marked his life since birth. In his home country of Morocco, he never learned to read or write, barely attended high school and communicated with his family through gestures, in an unofficial sign language, out of a necessity to be understood. Such was his life until a few twists and turns, an innovative gene therapy and several thousand kilometers brought him, with the help of Barcelona’s Hospital Sant Joan de Déu, to a children’s hospital in Philadelphia, where science offered Aissam his first access to the world of sounds. His mother, Naima Tbir, recalls with emotion how, just seven months ago, “the first day after the surgery, his father video called us with him and the child said to me [using signs]: ‘I’m hearing the sounds of the phone!’”
Aissam has become the first person in the Western world (Europe and the United States) to receive gene therapy to treat congenital deafness. In his case, it has been a success: he’s recovered his hearing and can identify sounds, though doctors warn that, given his age, it’s too late to learn spoken language, and he’ll probably never be able to talk fluidly.
In the colorful corridors of San Joan de Déu, Aissam shuffles his feet, crestfallen. He is angry. He has no desire to sit for photos, questions or medical examinations. He sulks while Doctor Oliver Haag, head of Otorhinolaryngology (ear, nose and throat specialty) at the Spanish hospital, inspects his right ear. “He’s angry because he wants to go to school, he doesn’t want to miss a single minute of class,” his mother explains through a translator. The world of hearing that has opened up to the boy, who is now 12 years old, has started a new chapter in which even school has become a different experience. “Aissam was born deaf. We found out because we have two older children who can’t hear or speak either. He went to school in Morocco for a year, but he didn’t understand anything,” says Naima.
The boy had an uncommon condition, a deafness caused by a mutation of the OTOF gene, which plays a key role in hearing. “It is an autosomal recessive mutation, which is why there are families with several affected children. Parents can be healthy carriers, but if the children get the mutation from both parents, they suffer from the disease,” says Haag. Only 20,000 people in Europe suffer from the condition. “The OTOF gene encodes otoferlin, a protein present in the cells inside the cochlea that transmits sound in the cochlear fluid,” says Haag. In a healthy ear, sound travels as airwaves until it reaches the snail-like shell of the cochlea. There, the waves are transmitted through the fluid that bathes the inside of this shell to the auditory nerve. Sensory cells with hairs (called hair cells) function as sensors of the vibrations caused by sound and are responsible for transmitting sound stimulus to the brain. “Otoferlin is one of those proteins that, if it does not work properly, prevents hair cells from transmitting the stimulus correctly. Nerve stimulation does not work. The chain is interrupted and information does not reach the brain correctly. But if that gene, that protein, is repaired, the whole chain works again.”
Aissam and his family moved to Barcelona in 2018. His father, who had migrated to Spain 19 years before, was able to complete a process for family reunification and the young boy, along with his mother and siblings, reunited with his dad. He returned to school and there, “thanks to the school staff and a Moroccan friend who has a son with a similar problem,” says Naima, they got him entered into a Sant Joan de Déu program for treating deafness.
Genetic testing confirmed his OTOF gene mutation and, as the boy continued with his hospital visits and tests, Haag’s team embarked on an international study to look into the history of his disease. That research planted the seed for the gene therapy they would later develop, and Aissam’s turned out to be a particularly paradigmatic case, a perfect fit for the scientific revolution that was taking shape.

“Aissam was a particular case because he had the mutation, he was deaf and he did not have a cochlear implant,” says Haag. That’s a less common profile in Western countries, where such pathologies are often treated in early infancy with an implant that allows for the recuperation of auditory ability. “In our field, the majority of patients have a cochlear implant, which excludes them from being able to receive gene therapy. They have to have a very rare mutation and still have cells functioning within their cochlea to make them a possible candidate,” says the otolaryngologist.
The boy fulfilled all of the requirements and, with the approval of his family and doctors, he embarked on the clinical trial that is seeking to approve a new genetic treatment designed to allow him to hear. Its technique consisted in introducing into his hair cells, through a non-pathogenic (non-harmful) virus that served as a “Trojan horse” according to Haag, the necessary instructions for producing the otorferlin protein. “There is defective genetic information that makes it so the protein doesn’t get produced correctly. So, what it does is genetic transference: it brings the correct genetic information to the nuclei of the cells and the defect in the protein production is repaired, so that from then on, it can be produced correctly. In so doing, hearing can be restored,” Haag says.
Aissam traveled with his father and Haag to the Children’s Hospital of Philadelphia where he underwent the new treatment. The surgery, which was carried out on only his right ear, lasted a few hours, but the young boy stayed in town for three months to undergo exhaustive follow-up testing.
According to his family, the boy began to perceive sounds quickly. Such as the noises that a smartphone emitted when he and his father made their first video call back to the rest of the family in Spain. Aissam’s mother says that, during one of the first moments in which he realized he could hear, the boy, “with a gesture that means thanks to God,” put his hands together, drawing a kind of bowl, and expressed through the gestures: “Now I am listening much better than before!”
One month after the surgery, results from audiometries and medical studies indicated its apparent success. “The first trial was mainly intended to check on the patient’s safety, that all the application devices were functioning, which is why we started with low doses. We didn’t know if the dose had been sufficient or if it would have to be increased. It was a bit experimental. The surprise has been that it has worked very well, he has already recovered in a short amount of time — less than a month — 80% of his hearing: in the low frequencies, he’s at a slight disadvantage, but in the high frequencies, he has normal hearing at 20 decibels. He has gone from profound deafness to nearly normal hearing. It’s a big change. Before, he didn’t hear anything and now, when you call him, he turns around, he hears the sound of a car and when he gets a haircut. He says these are things that he never perceived before,” says Haag.
Difficulties with language
The young boy already makes sounds and has even learned to say his name, but doctors caution that he probably will not be able to fully learn spoken language. Gene therapy has allowed him to leave his world of silence, but he’s arriving too late to learn how to speak. “It’s all about how after those first three years of life, the disconnection between his ear and brain, results get a lot worse because the brain busies itself with other tasks and the auditory channels don’t develop in the same way,” says Haag. The process is also influenced by how much the boy was stimulated beginning early on, because there are deaf patients “who can be locutionary and speak,” he adds. This is not Aissam’s case. “If that’s not done, there’s no language or understanding. And now he’ll have the auditory input, but if the connection between the cochlea and the brain is not worked on and he does not have integrated space on the hard disc in the brain’s cortex that is dedicated to hearing, what he hears and understands will improve, but it will not be enough. Although you will notice improvement,” says the doctor.
It’s already been noticed, in fact — by both the boy and everyone around him. Haag himself says that he’s “a very happy, very friendly child” and “although he hasn’t been worked with, hasn’t received hearing rehabilitation and hasn’t been taught,” he will learn. “He is now in school, he has sign language and he can also rely on his auditory sensations. And surely, although neuroplasticity is important during the first years of life, he will, throughout his life, continue to learn and interpret what he hears.” Aissam plays soccer, goes to the swimming pool and loves his school, a center that is designed for children with special educational needs in Santa Coloma de Gramenet. His mother is emphatic that “he is very happy.”
Aissam is the start of a therapeutic revolution that, once established, could change the paradigm for care of this type of patient or others who experience deafness due to other genetic mutations. As part of the same study, there is one more child being treated in Taiwan and two more who will be treated in the United States. Another investigation, this one being carried out by Chinese researchers, has yielded similar results through a related technique. Scientists seek to carry out this gene therapy on younger patients so as not to miss the window of opportunity when it comes to learning language during the first years of life, and to minimize deleterious impacts as much as possible.
Haag appears enthusiastic about these treatments’ potential, but is still cautious in reminding that, “This is a beginning with a gene in a single ear. We’ve got to manage expectations.” Long-term effects are still unknown, as are the optimal timetable and technique for applying the treatment. Above all, the doctor asks patients not to give up on other kinds of treatment, like the cochlear implant, in favor of waiting for a gene therapy that is, for the moment, only experimental: “Global prospects are good and very interesting, but they are not here yet,” he says.
Sign up for our weekly newsletter to get more English-language news coverage from EL PAÍS USA Edition
Tu suscripción se está usando en otro dispositivo
¿Quieres añadir otro usuario a tu suscripción?
Si continúas leyendo en este dispositivo, no se podrá leer en el otro.
FlechaTu suscripción se está usando en otro dispositivo y solo puedes acceder a EL PAÍS desde un dispositivo a la vez.
Si quieres compartir tu cuenta, cambia tu suscripción a la modalidad Premium, así podrás añadir otro usuario. Cada uno accederá con su propia cuenta de email, lo que os permitirá personalizar vuestra experiencia en EL PAÍS.
¿Tienes una suscripción de empresa? Accede aquí para contratar más cuentas.
En el caso de no saber quién está usando tu cuenta, te recomendamos cambiar tu contraseña aquí.
Si decides continuar compartiendo tu cuenta, este mensaje se mostrará en tu dispositivo y en el de la otra persona que está usando tu cuenta de forma indefinida, afectando a tu experiencia de lectura. Puedes consultar aquí los términos y condiciones de la suscripción digital.









































