Monkeypox circulated among humans for years before the 2022 outbreak
Scientists say DNA samples show a human enzyme has increased the virus mutation rate to ‘worrying levels’
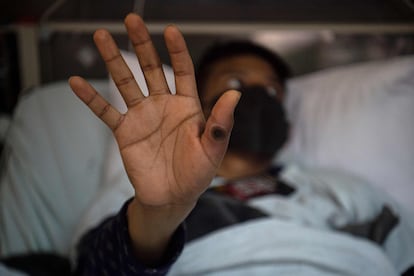
On April 29, 2022, a British citizen developed a rash while traveling in Nigeria. After returning to the U.K., he decided to go to the hospital as his rash got worse, lymph nodes swelled and fever rose. The doctors confirmed it was monkeypox, or mpox, as it was renamed by the World Health Organization (WHO). Monkeypox is a disease caused by a virus that usually spreads from animals to humans but rarely between humans. However, in just one month last spring, thousands of cases were reported worldwide and the WHO declared it an international public health emergency. Recent research by virologists has shown that the strain causing the outbreak had been circulating among humans for several years. They also found that a specific human enzyme with antiviral activity may have accelerated the mutation rate of the virus, raising concerns among scientists about the future of mpox.
The mpox threat has diminished now. While new infections persist, the WHO lifted its alert in the summer. However, the pathogen remains present, and its origin remains unknown. Historically, outbreaks typically involve transmission from animals to humans, often rodents or apes, with limited human-to-human transmissions. The virus did not effectively adapt to the human environment. However, something seemed to have changed in 2022. Within a few months, thousands of people got infected and by the end of October 2023, the number had already exceeded 91,000. Surprisingly, most of them had not traveled to countries like Nigeria or the Democratic Republic of the Congo, where mpox is typically found. This suggests that there was ongoing person-to-person transmission, which is not usually associated with a zoonotic (transmitted from animals) disease.
A group of scientists, including some who initially studied SARS-Cov-2, have recently sequenced the genome of nearly 100 mpox virus samples, some of which date back to the 1960s. The purpose of this research was to identify the origin of the B.1 strain responsible for the 2022 outbreak. This strain is categorized under clade IIb and originated in West Africa. Thankfully, it is about 10 times less lethal than clade II viruses found in Central Africa.
Their research study published in Science suggests that the 2022 samples are not the first instances of the outbreak. They share up to 42 mutations in their DNA, which were traced back to a 2015 case that already displayed one of these changes. In the following year, Nigerian authorities reported some cases of mpox in humans. Initially, they were believed to be of zoonotic origin and unrelated to each other. However, the researchers concluded that there had been “sustained human-to-human transmission” since 2016.
“It’s not clear what led to the global spread of B.1. There doesn’t seem to be anything particularly different about this lineage of the virus.”Áine O’Toole, virologist, University of Edinburgh (U.K.)
To support this conclusion, they pointed to the origin of the 42 changes in the viral DNA, focusing on nucleotides — the fundamental elements of DNA (A, T, G and C). They found that nearly all of these mutations are associated with an enzyme called APOBEC3, which is present in most mammals. Rodents, known as virus reservoirs, only have one copy of this enzyme in the spleen and bone marrow, not in other tissues. In humans, the enzyme is part of the immune system and helps remove parts of the virus’s DNA that hinder replication. These genetic modifications emerged after 2017, indicating that they did not occur prior to the virus transmission from animals to humans.
The lead author of the research study, virologist Áine O’Toole from the University of Edinburgh (U.K.), said, “Since it began affecting humans in 2016, the changes made to the APOBEC3 gene are visible as scars on the virus genome.” When viruses replicate, they expose their DNA, which allows this enzyme to replace certain letters with others. This interference affects the replicative machinery in most cases, but sometimes the pathogen still manages to replicate, now marked with APOBEC3. However, O’Toole doesn’t have an answer for why the outbreak occurred in May 2022 after circulating for at least six years. “It’s not clear what led to the global spread of B.1. There doesn’t seem to be anything particularly different about this lineage of the virus. It probably spread widely because it entered specific population networks.”
Antonio Alcamí, a virologist with Spain’s Severo Ochoa Molecular Biology Center (CMB/CSIC), underscores the importance of knowing when it began to circulate: “It was previously considered to be of recent origin. However, the same strain of the virus responsible for the 2022 outbreak has been detected in humans since 2016.” Alcamí (who did not participate in this study) says these latest findings are very significant. “It was believed that mpox did not infect humans, but we now know otherwise, which serves as a warning that mpox is indeed adapting to humans.”
“The longer the virus circulates among people, the greater the likelihood that it will adapt to the human body.”Raúl Rivas, professor of microbiology and genetic, University of Salamanca (Spain)
One possible factor could be that APOBEC3 has accelerated mpox’s mutation rate. Compared to previous orthopoxviruses like human smallpox, which had a very slow rate of change, mpox displayed 42 DNA changes in two to three years. This is a significantly faster rate — 28 times faster — than previous mpox lineages. “The main point is to determine whether these mutations increase transmissibility among humans,” said Raúl Rivas, a professor of microbiology and genetics at the University of Salamanca (Spain). Rivas also stresses the importance of dating the first cases with these mutations. “The longer the virus circulates among people, the greater the likelihood that it will adapt to the human body.”
The authors of the study clarified that although the antiviral enzyme is responsible for the mutations in the B.1 lineage, it does not necessarily imply that APOBEC3 is boosting the virus’s ability to replicate and transmit among humans. Fernando González, a professor at the University of Valencia (Spain) who studies phylogenomics (reconstructing the evolutionary histories of organisms), notes, “APOBEC3 does not directly cause mutations, but it is mutagenic. Some of these mutations have increased the virus’s transmissibility.” The next urgent step is to link the antiviral enzyme with this increased capacity for contagion.
The study concludes that if a connection between APOBEC3 and the long-term presence of mpox in humans is discovered, it would represent a significant shift in our understanding of the virus. Oriol Mitjà, from the Germans Trias i Pujol Hospital in Barcelona, participated in the discovery of a necrotizing form of mpox in people with advanced HIV. “We can expect new zoonotic outbreaks of mpox in the future. However, if transmission between humans persists, we can consider it a human virus. This represents a paradigm shift, much like we saw with HIV [which was initially zoonotic],” said Mitjà.
The recent findings have heightened concern about the spread of mpox. The majority of the over 91,000 individuals infected so far are under the age of 50, implying that they have not received the smallpox vaccine (smallpox was eradicated in 1980). There is a potential risk that mpox could fill the void left by its viral relative and spread within an unimmunized population. The situation could become even more dire if the B.1 lineage successfully transmits between humans, as clade I viruses are fatal in 10% of the cases.
Sign up for our weekly newsletter to get more English-language news coverage from EL PAÍS USA Edition
Tu suscripción se está usando en otro dispositivo
¿Quieres añadir otro usuario a tu suscripción?
Si continúas leyendo en este dispositivo, no se podrá leer en el otro.
FlechaTu suscripción se está usando en otro dispositivo y solo puedes acceder a EL PAÍS desde un dispositivo a la vez.
Si quieres compartir tu cuenta, cambia tu suscripción a la modalidad Premium, así podrás añadir otro usuario. Cada uno accederá con su propia cuenta de email, lo que os permitirá personalizar vuestra experiencia en EL PAÍS.
¿Tienes una suscripción de empresa? Accede aquí para contratar más cuentas.
En el caso de no saber quién está usando tu cuenta, te recomendamos cambiar tu contraseña aquí.
Si decides continuar compartiendo tu cuenta, este mensaje se mostrará en tu dispositivo y en el de la otra persona que está usando tu cuenta de forma indefinida, afectando a tu experiencia de lectura. Puedes consultar aquí los términos y condiciones de la suscripción digital.









































