Chronic inflammation: How to prevent the body from living on constant alert
Fifty-percent of global mortality can be attributed to diseases related to this condition, but what exactly is it and what causes it?
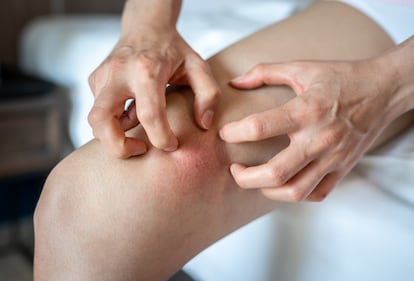
When we get hit or bitten by a mosquito, we can see the external part of an inflammation process with our own eyes. The affected area turns red and hot, it swells and it hurts or itches; if all goes well, it will recover its normal appearance in a few days. The same thing happens when the inflammation is caused by an infection – in the throat, for instance – or if we sprain an ankle: that is where you notice it, not on a shoulder. In all these cases, we see and feel an inflammation in a specific area.
However, it is increasingly common to come across headlines that warn of another type of inflammation, one that is harder to understand. We read that certain foods or diets cause inflammation; stress or lack of sleep do too. This is a negative inflammation, related to multiple diseases, something we would certainly be interested in avoiding. But is it related to the inflammation we get from an injury, or is this something entirely different? What part of the body becomes inflamed when, for instance, we sleep badly on a continuous basis?
The answer to the first question is yes. Inflammation is a set of physiological responses that organisms use to try to maintain stability (also known as homeostasis), explains Jaime Millán, senior scientist at the Spanish National Research Council (CSIC). That stability comes from maintaining certain variables such as blood pressure, temperature or glucose levels within an “acceptable” range, something for which we have autonomous mechanisms. It is when these mechanisms are not able to maintain this regulation that the inflammatory response appears. This is mainly “a protection and defense mechanism,” says Millán, who is also deputy director of the Severo Ochoa Center for Molecular Biology (CBM) at the Autonomous University of Madrid, in Spain.
Researcher and nutritionist Pedro Carrera-Bastos, co-author of a study published in the journal Nature on the relationship of multiple diseases with chronic inflammation, is clear on this point: even if we are paying attention to the bad part of inflammation — for example, when it becomes chronic — the process itself is essential for our survival. “The problem would be not being able to undergo an inflammatory process. If our ancestors had not been able to activate an inflammatory response whenever it was needed, we would probably be extinct as a species,” he points out.
The problem comes when inflammation does not work properly, it does not go away after the problem is solved or when, being exposed to constant inflammatory stimuli, it extends over time. Marcos López Hoyos, president of the Spanish Society of Immunology (SEI), speaks of control and regulation as key elements in this transition from good to bad inflammation: “When it is not well controlled — either its duration or its magnitude — the inflammation goes too far and produces diseases,” he explains.
From localized to systemic
Going back to the question of what exactly is becoming inflamed when the origin is not a bug bite but, for instance, constant stress, Jaime Millán explains that the local inflammatory signals sent by the cells of the immune system that are close to the bite (for example) can other times be induced systemically, throughout the entire body. This can happen due to an infection (here we would be talking about sepsis), but also “progressively, but constantly, in response to stress or unhealthy lifestyle habits,” says Millán. In this type of inflammation, our body is in a permanent state of alert, issuing danger signals and sending immune cells throughout the entire organism in response.
To better understand how and why this happens, let’s follow the path of inflammation, from the blow to the throbbing bump. Faced with a problem or a threat, who gives the alarm? The main sensors of these stress signals (infections, broken tissues, blows, etcetera), which can trigger an inflammatory response are, on one hand, the cells of the immune system found in the tissues, responsible for a more primitive type of immunity, known as innate; and on the other hand, the sensory neurons, which detect various types of external stimuli, elaborates Millán. All these cells, which saw the threat firsthand, trigger inflammatory mediators that activate the rest of the necessary cells to “orchestrate the inflammatory response.”
Here is an important detail: neither these mediators nor the rest of the cells that are activated have seen that initial threat. That is to say, whoever sees the problem (the molecules of a bacterium, for example) emits numerous messages that reach the rest of the cavalry, which springs into action. “The initial activation is amplified and activates many more cells, especially the innate immune system,” says Millán.
From acute to chronic
In the example of a bite or a blow, the inflammation, in addition to being localized, is usually acute and transitory. It starts quickly and is over in a short period of time. When this does not happen, due to a lack of control or because we keep constantly receiving these inflammatory stimuli, the inflammation becomes chronic.
This can happen because the aforementioned messengers, mediators such as inflammatory cytokines, are also secreted by cells “that are under some type of stress or a deregulation of their natural balance or homeostasis,” points out Millán. As an example, he mentions the fat-filled adipocytes in the adipose tissue. “If the fat storage capacity of these cells exceeds a certain threshold, a stress response occurs that triggers the secretion of inflammatory cytokines in a chronic, rather than acute, manner,” he explains. “The cells of the immune system throughout the entire body are chronically activated, as if we had a very weak but persistent infection.”
This chronification ends up producing diseases. Millán provides another example: “High cholesterol levels, a sedentary life, etcetera, cause lipids to accumulate inside the walls of the blood vessels. This generates the secretion of inflammatory signals that attract immune cells that migrate to these places of lipid accumulation as if it were a focus of infection. However, instead of doing it temporarily, they do so slowly and steadily and accumulate inside the vessel in a process that takes years, contributing to the formation of atherosclerotic plaques that can end up blocking the bloodstream,” he explains. If another source of chronic emission of inflammatory mediators is added to this, this arteriosclerosis will accelerate, he adds. These sources can be the aforementioned adipocytes or, for example, “a mouth in very poor condition that is full of bacteria, and therefore, always inflamed.”
It is this chronic type of inflammation the one that all those articles full of advice refer to. “Currently, a lot of attention is being paid to low-grade systemic chronic inflammation,” says Carrera-Bastos. According to the nutritionist and researcher, the interest is due, on one hand, to the fact that this type of inflammation does not usually produce signs or symptoms, which is why in many cases it goes unnoticed. In addition, this chronic inflammation is noteworthy because it is related to so many diseases: “Type II diabetes, non-alcoholic fatty liver disease, chronic kidney disease, cardiovascular diseases, various types of cancer, sarcopenia, osteoporosis, osteoarthritis, depression and neurodegenerative diseases,” list the expert.
López Hoyos agrees: “We immunologists are doing a lot of research right now because we are seeing that, when that part is deregulated, this pathological inflammation occurs, which has a lot to do with the disease and which alters all the components of the adaptive immune response that we studied before,” he says.
Nutrition in the spotlight
Taking into account that 50% of the deaths that occur in the world are attributable to diseases related to inflammation, it makes sense to want to do everything possible to prevent or reduce it. That explains all those articles that extol some food as an almost magical antidote – often with little scientific basis. Is there really any food that can save us from chronic inflammation? “Before worrying about which foods, nutrients, bioactive compounds and diets are capable of reducing or curing inflammation,” Carrera-Bastos recommends looking at the main promoters of a low-grade chronic inflammatory state and trying to act there first: pollution, tobacco, inadequate sleep, psychological stress, physical inactivity, obesity and the wrong diet.
“In this sense, and according to various intervention and epidemiological studies, non-pharmacological interventions that prevent, reduce or cure inflammation are smoking cessation, some stress control strategies (such as yoga, tai chi, meditation or just spending more time in green spaces), adequate sleep, physical exercise, decreased body fat (especially visceral fat, which is located mainly in the abdominal cavity along with organs like the liver and the pancreas) and a healthy diet that provides all the nutrients necessary,” he lists. In other words, before looking for anti-inflammatories, it is better to eliminate the sources of inflammation.
After taking measures in these lifestyle aspects, you can delve further into specific “nutritional strategies.” That is, rather than superfoods with quasi-magical qualities, it is more convenient to simply focus on food in general. “There are diets that promote inflammation and others that are associated with a decrease in these inflammatory mediators in patients, of which of course the Mediterranean diet is the best example,” says Jaime Millán.
Numerous research studies on inflammation are focusing precisely on the area of nutrition, explains Millán, who is confident that in the coming years the intestinal bacteria will be described in detail based on different types of foods and their effect on the immune system. The effect of different types of diets on the composition of the intestinal microbiota will also become known, he assures.
As an example, he explains that a diet rich in ultra-processed foods “alters and decreases the diversity of the microbiota,” as these foods do not need as much bacteria to be digested and absorbed, which causes the nutrients available for their survival in the intestine to decrease. This has a pro-inflammatory effect, as the lower the population of beneficial bacteria, the greater the possibility of being colonized by pathogenic bacteria that signal the immune system and produce inflammation, he explains.
Regarding the nutrients that seem to be emerging as ideal when it comes to fighting inflammation, Carrera-Bastos mentions omega-3 fatty acids, “which have already been shown to reduce several analytical parameters of inflammation and improve the clinical picture of some chronic inflammatory diseases, such as rheumatoid arthritis.” Still, before you stuff yourself full of sardines, mackerel and salmon, it is worth remembering his first piece of advice: to look at the rest of the inflammatory promoters and try to reduce them as much as you can. Sardines, after all, are not some magic bullet that will neutralize all the hours we spend sitting down or the effects of living under continuous stress.
Sign up for our weekly newsletter to get more English-language news coverage from EL PAÍS USA Edition
Tu suscripción se está usando en otro dispositivo
¿Quieres añadir otro usuario a tu suscripción?
Si continúas leyendo en este dispositivo, no se podrá leer en el otro.
FlechaTu suscripción se está usando en otro dispositivo y solo puedes acceder a EL PAÍS desde un dispositivo a la vez.
Si quieres compartir tu cuenta, cambia tu suscripción a la modalidad Premium, así podrás añadir otro usuario. Cada uno accederá con su propia cuenta de email, lo que os permitirá personalizar vuestra experiencia en EL PAÍS.
¿Tienes una suscripción de empresa? Accede aquí para contratar más cuentas.
En el caso de no saber quién está usando tu cuenta, te recomendamos cambiar tu contraseña aquí.
Si decides continuar compartiendo tu cuenta, este mensaje se mostrará en tu dispositivo y en el de la otra persona que está usando tu cuenta de forma indefinida, afectando a tu experiencia de lectura. Puedes consultar aquí los términos y condiciones de la suscripción digital.








































