Two synthetic biology experiments offer hope for future cancer treatments
New immunotherapies can allow healing cells to be activated or deactivated according to the stage of the disease, while more accurately targeting tumors
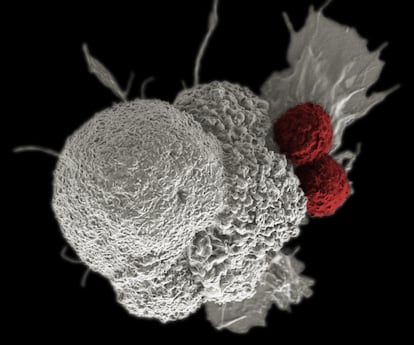
Cancerous cells, despite all the harm they cause, are also ours – it’s difficult to distinguish them from healthy ones. This is why chemotherapy – which began in the mid-1950s – kills off both healthy and diseased cells, in the hope that the body will be able to regenerate healthy ones. Over time, targeted treatments arrived, which sought to block the expression of specific proteins, thus stopping the progression of the disease.
In recent years, immunotherapies such as CAR-T have taken treatment personalization to a new level. Chimeric cells are created after extracting a patient’s T lymphocytes – the specialized cells of the immune system – introducing modifications to them (so that they recognize specific antigens on the surfaces of tumors) and injecting them again. These chimeric cells subsequently target and eliminate cancerous cells.
There are, of course, limits to this. With each new treatment, new side effects arise, while cancer maintains its capacity to adapt and outwit treatments over time. CAR-T therapies have shown efficacy with liquid tumors, such as leukemia, but have not been able to overcome the immunosuppressive barriers that deactivate them before they reach solid tumors, such as pancreatic tumors or melanoma. Additionally, these treatments cannot be quickly modified in response to the rapid adaptive capacity of cancer cells.
This past week, two research teams published articles in the journal Science. The authors propose solutions that rely on synthetic biology to overcome some of the aforementioned limitations. It must be noted that these technologies have, so far, only been tested on mice.
In one of the experiments that was conducted, a team led by Ahmad Khalil, from Boston University, developed a series of genetic switches that can be added to human immune cells. These switches make it possible to regulate the cells, starting their antitumor activity in a specific moment. The scientists designed the switches to be activated by cancer drugs that have already been approved by the FDA, such as grazoprevir – which is used to treat hepatitis C – or afimoxifene, which is used to treat breast cancer.
“Right now, T lymphocytes are not capable of adapting to defeat the tumors,” says Emanuel Salazar-Cavazos, a Mexican researcher at the National Cancer Institute in Bethesda, Maryland. He is a co-author of an analysis of the findings, which has also been published by Science.
“[With these new techniques], at any given moment, if a sample is taken and you see that those T lymphocytes are losing the battle, you could modify some genes to regulate a function. This allows us to counteract the mechanisms that the cancer cell is using to evade the immune system,” he continues.
This technology would allow immunotherapies such as CAR-T to be adapted to a disease as dynamic as cancer. Grégoire Altan-Bonnet – the leader of Salazar’s team – points out that “these cells could be modified so that there are several populations – some active and others at rest – that can change their state, so that there are always some active ones ready to attack tumor cells.”
As explained by Luis Álvarez-Vallina – director of the National Cancer Research Center in Madrid – this tactic of switching cells on and off would prevent “lymphocytes from becoming exhausted […] something that happens when they act continuously.” He also explains that it would be possible to deactivate the CAR-T therapy if a patient is suffering from negative side effects.
In the second experimental study, a team led by Wendell Lim, from UC San Francisco, added synthetic receptors to CAR-T cells, thus refining the timing of the attack of the improved lymphocytes. This served for the CAR-T to produce interleukin 2 – a protein that triggers the immune attack, but only when there is direct contact with the tumor cells.
On the one hand, this technology reduced the collateral damage of the immunological bombardment. And on the other, it made it possible to overcome the immunosuppressive environment that protects solid tumors. The results saw reduced tumor size in the mice, who served as test subjects.
If these techniques work on human beings, researchers will be able to adapt cancer therapies in a way that will focus attacks specifically on cancerous cells without injuring patients. Lim explains that they are already “planning to use this technology in clinical trials” within a year or two. Khalil notes that the technology is not quite ready for human trials, but they hope to partner with biotech firms to begin this new phase in the future.
For these types of treatments to eventually reach many people, they must overcome the cost of producing them. Currently, CAR-T therapies cost hundreds of thousands of dollars – the addition of synthetic biology is not going to make them any cheaper in the short-term. In the long-run, however, Altan-Bonnet says that cost-reduction is possible: “One of the big problems with CAR-Ts is that you have to do a lot of them, because they have weak activity. With the technology that has been mentioned in these articles, the quality could be improved and, perhaps, it would not be necessary to produce as many. This would make the process cheaper.”
Álvarez-Vallina believes that these improved treatments will reach most cancer patients “relatively quickly, either via these synthetic biology technologies or similar ones.” However, he does not think it is likely that these advanced cell therapies will completely replace the treatments used today, such as chemotherapy or targeted drugs.
“In the far future, there will be increasingly personalized treatments and new technologies will be incorporated, but the combination of techniques will be very important, including the ones we use now.”
Sign up for our weekly newsletter to get more English-language news coverage from EL PAÍS USA Edition
Tu suscripción se está usando en otro dispositivo
¿Quieres añadir otro usuario a tu suscripción?
Si continúas leyendo en este dispositivo, no se podrá leer en el otro.
FlechaTu suscripción se está usando en otro dispositivo y solo puedes acceder a EL PAÍS desde un dispositivo a la vez.
Si quieres compartir tu cuenta, cambia tu suscripción a la modalidad Premium, así podrás añadir otro usuario. Cada uno accederá con su propia cuenta de email, lo que os permitirá personalizar vuestra experiencia en EL PAÍS.
¿Tienes una suscripción de empresa? Accede aquí para contratar más cuentas.
En el caso de no saber quién está usando tu cuenta, te recomendamos cambiar tu contraseña aquí.
Si decides continuar compartiendo tu cuenta, este mensaje se mostrará en tu dispositivo y en el de la otra persona que está usando tu cuenta de forma indefinida, afectando a tu experiencia de lectura. Puedes consultar aquí los términos y condiciones de la suscripción digital.









































