Microbe study sheds light on a critical step in the evolution of life on Earth
A ‘living fossil’ reveals how the original ancestor of all animals, plants and fungi could have come into being 2.5 billion years ago
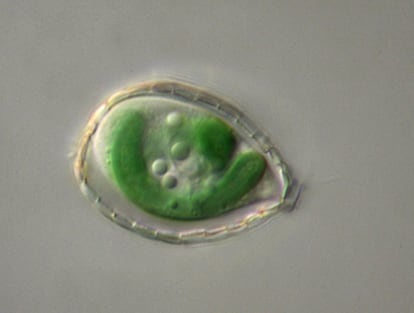
A research study on a microscopic microbe found in lakes and seas today is helping scientists understand one of the most important chapters in the evolution of life on Earth – the appearance of complex cells 2.5 billion years ago.
All animals, plants and fungi are made up of several eukaryotic cells, which have internal organelles unlike unicellular bacteria and archaea. Most scientists think that they originated as a result of one microbe swallowing another. But instead of simply digesting the swallowed microbe, an interdependent relationship spontaneously developed: the larger microbe provided protection from external threats, and the smaller microbe provided food. Two and half billion years of interdependent evolution later, thousands of mitochondria related to that original devoured microbe float inside each of our cells and transform food into the energy needed by the brain to think, and by the heart to beat. Thanks to chloroplasts – another type of organelle – plants can feed on light through photosynthesis.
The appearance of the modern cell “was a revolution for the evolution of life on Earth,” says Victoria Calatrava, a biochemist at the Carnegie Institution for Science in the United States. “Without it, our planet would not look anything like what we see today, nor would we be here to tell the tale.” The 32-year-old Spanish scientist from Córdoba aims to demonstrate exactly how that union happened. It is a difficult problem to solve because time has almost completely diluted all the molecular traces.
Calatrava leads a research study of the origin of our cells that examines an aquatic microbe called Paulinella micropora. This organism is the union of an amoeba that devoured a cyanobacterium, which is a microbe capable of photosynthesis.
This type of endosymbiosis has only occurred twice in the history of evolution. The first time was 1.5 billion years ago and gave rise to the chloroplasts that provide energy to plants. The second time produced Paulinella a mere 120 million years ago. It’s called a living fossil because many of the traces that evolution erased in other organisms are still visible in this amoeba.

The Proceedings of the National Academy of Sciences (PNAS), the Academy’s official journal, published a study on Tuesday in which Calatrava’s team explain how the union of two such different living things happened. This could also explain what happened 2.5 billion years ago when mitochondria appeared in the first complex cell.
Photosynthetic bacterium began to shed genes that landed in the genome of the host amoeba. A process known as retrotransposition then caused some genes to be copied many times over and enabled them to function more efficiently. Scientists have shown that this adaptive process enabled the amoeba to enhance its genes so that it could tolerate toxic compounds associated with photosynthesis, compounds that would have otherwise killed the amoeba during attachment.
It was an irreversible step. The bacterium shed so many genes that it could no longer survive on its own, and the metabolism of the host amoeba changed so much that it could no longer be a predator. “Both benefit from each other’s existence, and they are completely interdependent,” said Calatrava, who advises us not to regard this as a benign process. “I don’t see it as a win-win, cooperative relationship. Rather, they are forced to keep each other alive so that they don’t become extinct.”
This compulsion to share resources continues to characterize life on Earth. A human being is composed of 30 trillion human cells, and has another 39 trillion bacteria living in its digestive system. Without these bacteria, humans would not be able to digest food. In return, bacteria are able to exist in an environment with fewer predators than they would encounter outside the body. Nothing prevents these relationships from breaking down, as is often the case with antibiotic-resistant bacterial infections. These are organisms that are posing a medicine exchange resistance mechanism by using a gene transfer process similar to that seen in the Paulinella amoeba. “The results of our research suggest that this mechanism has played a crucial role in the domestication of foreign genes, in the context of endosymbiosis. It seems very likely that it has also played a key role in the stabilization of endosymbionts, and the evolution of organelles in other systems,” said Calatrava.
Juli Peretó, an expert in synthetic biology at the University of Valencia in Spain, emphasizes the importance of this study. “Life on Earth first appeared about 3.5 billion years ago in the form of bacteria and archaea. These life forms invented respiration and photosynthesis. A billion years later, an archaeon phagocytosed a bacterium and complex cells emerged. Another billion years later, a new union between photosynthetic bacterium and a different microbe appeared, giving rise to the chloroplasts that carry out photosynthesis in plants. This study of Paulinella provides a standalone framework that enables us to reconstruct the story of how it all began,” said Peretó.
“It makes sense that everything happened the way this study hypothesizes, although it is difficult to say for sure,” says Toni Gabaldón, a biologist and researcher at the Center for Genomic Regulation in Barcelona, Spain. Gabaldón believes that the exchange of genes between the bacterium and the amoeba was a process of accelerated improvement, like a poker player who discards a card and draws a new one that turns a lowly pair into a winning hand. The only thing that remains to be demonstrated is whether this lucky card draw can explain the origin of all eukaryotes or only those that carry out photosynthesis.
Paulinella has a clear competitor in this quest to clarify the origins of our cells: the Asgard archaea. Two years ago, Japanese scientists announced that they had finally succeeded in breeding these creatures outside their deep-sea habitat. Researchers consider it another living fossil. These organisms measure one ten-thousandth of a centimeter and reproduce about once a month, very slowly by microbial standards. Their most striking feature is their long, intertwined tentacles. Scientists do not yet know how they use these tentacles, but believe they are essential to explaining how complex life arose from very similar organisms.
“The discovery of the Asgard archaea will be of great help in understanding the origin of complex cells,” says Iñaki Ruiz-Trillo, a researcher at Spain’s Institute of Evolutionary Biology. “I am optimistic and believe that we will succeed in clarifying this issue. We know much more now than we did 20 years ago, and we will know even more in time. One day, a new basal eukaryote or a new archaeon may provide clues that we can’t even imagine right now.”
Tu suscripción se está usando en otro dispositivo
¿Quieres añadir otro usuario a tu suscripción?
Si continúas leyendo en este dispositivo, no se podrá leer en el otro.
FlechaTu suscripción se está usando en otro dispositivo y solo puedes acceder a EL PAÍS desde un dispositivo a la vez.
Si quieres compartir tu cuenta, cambia tu suscripción a la modalidad Premium, así podrás añadir otro usuario. Cada uno accederá con su propia cuenta de email, lo que os permitirá personalizar vuestra experiencia en EL PAÍS.
¿Tienes una suscripción de empresa? Accede aquí para contratar más cuentas.
En el caso de no saber quién está usando tu cuenta, te recomendamos cambiar tu contraseña aquí.
Si decides continuar compartiendo tu cuenta, este mensaje se mostrará en tu dispositivo y en el de la otra persona que está usando tu cuenta de forma indefinida, afectando a tu experiencia de lectura. Puedes consultar aquí los términos y condiciones de la suscripción digital.









































