Little Owen’s big milestone: the piece of heart transplanted to cure his heart failure has grown along with him
The first partial heart transplant on a newborn with severe vascular dysfunction proves successful: the organ works, and the implanted valves and vessels have developed along with the child
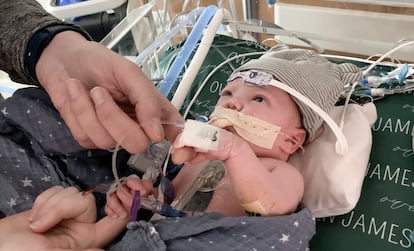
Owen Monroe’s heart was born sick. The little one suffered from an extremely complex and rare cardiopathy; if it wasn’t repaired immediately, it would lead to his death. His aorta and pulmonary arteries were fused, and he had only one other valve (healthy hearts have aortic and pulmonary arteries, plus the mitral and the tricuspid valves) to control the passage of his blood. Time was running out, his heart was struggling with each passing moment and his doctors at Duke University Hospital (North Carolina) were desperately studying their options. Monroe was even put on the list for a heart transplant, even though they suspected that he wouldn’t become eligible: he had just been born and already suffered from severe respiratory failure that put his future in jeopardy. “Every day seemed to last an eternity. With each passing day, you could feel the wait weighing down on the ICU staff. It was tense for everyone having to stand by day after day with no news, knowing that Owen was slowly dying,” remembers Tayler Monroe, the little boy’s mother.
The traditional solution when it comes to newborns with irreparable heart valve dysfunction is usually to replace the failures with artificial or cadaveric donor valve implants, but these tissues come with a major drawback: as they are inert grafts, they do not grow with the patient. As the child ages, they have to be replaced with new surgeries to adapt them to the size of the heart. In Owen’s case, however, his doctors wanted to try a new technique: a partial heart transplant, the first in the world, which would implant living tissue in order to avoid successive operations to replace his valves. The family agreed to this novel approach, and in the spring of 2022, when he was just 18 days old, surgeons transplanted part of a donor heart into the baby to reconstruct the affected vessels and valves. The procedure was a success. So much so, that a study by his doctors published in the journal Jama, put out by the American Medical Association, shares that his heart is working properly and the implanted tissue is growing with him, just as his doctors had hoped.
However, in Owen’s case, problems in the embryonic development of the large arteries of his heart had been detected during prenatal checkups. “Owen had a rare congenital hart defect called truncus arteriosus. Instead of having two arteries and valves coming out of the heart, he had only one, and that valve leaked terribly. We knew this from prenatal ultrasounds before he was born, so we were able to prepare for this groundbreaking procedure before his birth,” says Joseph Turek, chief of pediatric cardiac surgery at Duke and one of the architects of the partial heart transplant.
The medical team knew that the traditional approach, with valve replacements via artificial or cadaveric donor grafts, was not without additional risks, and would inevitably lead patients to more future interventions because the tissue would not grow. “Traditionally, there is an initial 50% mortality rate with this approach, and an additional 15% mortality every year thereafter, because of the need for numerous, risky operations to replace the non-growing valves,” says Turek. So, they opted to try a novel alternative that they arrived at out of their own clinical experience with heart transplants, the physician says via email. “Valves and arteries grow when we do whole heart transplants, so why wouldn’t just the valves and arteries grow if they were obtained from a donor and the recipient patient received some level of anti-rejection drugs?” he wondered. And they went for it.
With the family’s approval, and as soon as they had an organ available from a donor that was suitable for the case — Turek emphasizes that it had to be a heart that was not suitable for a complete transplant — they implanted part of it in Owen. “This involved sewing together both the new aorta (with the aortic valve) and the pulmonary artery (with the pulmonary valve). We also had to reimplant the coronary arteries into the new aorta and close the hole between the pumping chambers of the heart,” explains the doctor.
Outside the operating room, the wait seemed endless for Tayler and Nick Monroe. “It was the longest nine hours of our lives. We tried to eat pizza, but we couldn’t. We just sat there, looking at the clock in the operating room. We were just sitting there, looking at the clock in the [hospital] lobby,” Tayler says via email. Every hour or half-hour, they would get a call from the scrub nurse to let them know how the operation was going, and around midnight, the doctors confirmed that everything had gone well.
The operation was indeed a success, and one month later, the boy left the hospital in the arms of his parents. Subsequent follow-up over the course of the next year proved that the transplanted arteries and valves were working perfectly and, moreover, growing as if they were Owen’s own. “This is the first demonstration that a valve implant can grow in a human being. This solves a number of problems we face in children who need tissues that grow with them, and can save countless risky reoperations in these infants and children,” states Turek.
Lifelong valves
In the absence of long-term studies on the evolution of Owen and other children with partial heart transplants, researchers theorize that while the long-term outcomes of a complete heart transplant are limited, with the organ eventually failing, “[outcomes] are limited by the inevitable ventricular dysfunction,” as the article on Owen states, “partial heart transplants respect native ventricles and are therefore expected to last a lifetime.”
Carlos Velasco, cardiovascular surgeon at the A Coruña University Hospital Complex (CHUAC), points out that this therapeutic approach “is of interest” in a specific situation: that of discarding a donor organ because it is not suitable for a complete transplant. “Neonatal transplantation is an absolute rarity; it is very difficult to carry out these transplants because there are no organs. So, when you have obtained an organ that does not work, but the valves do work, it is interesting to be able to use part of an organ that would otherwise not be utilized,” says Velasco.
Turek confirms that effectively, in Owen’s case, “the [donor’s] heart was not suitable for a full heart transplant.” “We do not use hearts from the waiting list for full heart transplants. In the first place, the hearts should be considered unsuitable for a full heart transplants,” says the U.S. physician. Of all the hearts that are donated, the physician estimates, only half meet the criteria to be used for a full transplant, but the other 50% could potentially be used to take advantage of their valves, he says.
Velasco, who did not participate in the study, also believes that resorting to an optimal donor heart to use only the valves would make sense, at least, “if it were not necessary to give immunosuppressants” to the patients. These drugs, which are administered to transplant recipients to prevent the recipient’s body from rejecting an organ received, also have their long-term risks, warns the CHUAC cardiac surgeon: “There is a risk of neoplasms [cancer] derived from immunosuppression and also infections.”
Turek has limited the use of his technique to a very specific patient profile and advocates for lower doses of immunosuppressants: “Most complete heart transplants are necessary because the heart muscle does not function well. Partial heart transplantation is for patients with valvular problems. Fortunately, the amount of immunosuppression needed in these cases appears to be only a quarter of that used in whole heart transplants. This is a non-life-altering dose.”

For his part, cardiologist Ferran Gran, coordinator of pediatric heart transplantation at Barcelona’s Hospital Vall d’Hebron, considers this new therapeutic approach “very novel, interesting and promising”. “It is novel because in these pathologies, when denatured valves are inserted, they tend to dysfunction and do not grow: the child grows and the graft does not. And that means that you have to reoperate many times. Theoretically, and this is what we are seeing right now, this can greatly extend the life of the graft,” says the cardiologist, who also did not participate in the study. Gran also believes that this technique “is a better alternative that the previous option” and adds that it would even be “applicable to other patients with less serious pathologies.”
Domino partial heart tranplants
According to Turek’s calculations, 13 partial heart transplants have already been performed worldwide, nine of them at Duke Children’s Hospital. The physician says that his technique “has opened several more doors to maximize donations to help children in need.” He refers to two new approaches within the dynamics of transplantation: the so-called domino partial heart transplants and split-root partial heart transplants.
With them, a single organ can wind up saving two lives. “In the domino partial heart transplant, you have a child who receives a full heart transplant. What happens to the child’s old heart? It’s usually discarded. However, if the patient consents to donate his or her old heart when it is removed, the valves usually work well. These valves can then be used in another child as a partial heart transplant. To date, five of these domino partial heart transplants have been performed, three of them here at Duke. For split-root partial heart transplants, a donate heart has two valves emerging from it (aortic and pulmonary). If you split these two roots, you can perform a partial heart transplant on two different children who only need one valve each — one heart helping two children! Two such cases have been performed at Duke,” says Turek.
A few months shy of the two-year anniversary of Owen’s groundbreaking operation, the little boy’s heart is beating strong. “He’s doing great! He has some developmental delays that are common in cardiac babies, but he walks and can feed himself. He loves investigating everything, opening and closing doors … He really loves his second chance at life,” Tayler says. Their son left the hospital taking 17 prescriptions a day, but now he takes only two and requires no special care. He’s just “a normal kid on some medications,” Tayler says. She adds, “With traditional truncal repair, Owen would have already had to have three open heart surgeries to change out valves as he grew older, but he won’t have to have any for the rest of his life if all goes well.”
Sign up for our weekly newsletter to get more English-language news coverage from EL PAÍS USA Edition
Tu suscripción se está usando en otro dispositivo
¿Quieres añadir otro usuario a tu suscripción?
Si continúas leyendo en este dispositivo, no se podrá leer en el otro.
FlechaTu suscripción se está usando en otro dispositivo y solo puedes acceder a EL PAÍS desde un dispositivo a la vez.
Si quieres compartir tu cuenta, cambia tu suscripción a la modalidad Premium, así podrás añadir otro usuario. Cada uno accederá con su propia cuenta de email, lo que os permitirá personalizar vuestra experiencia en EL PAÍS.
¿Tienes una suscripción de empresa? Accede aquí para contratar más cuentas.
En el caso de no saber quién está usando tu cuenta, te recomendamos cambiar tu contraseña aquí.
Si decides continuar compartiendo tu cuenta, este mensaje se mostrará en tu dispositivo y en el de la otra persona que está usando tu cuenta de forma indefinida, afectando a tu experiencia de lectura. Puedes consultar aquí los términos y condiciones de la suscripción digital.









































