New Covid-19 treatments strengthen medicine’s fight against the pandemic
A new range of oral antivirals by Pfizer and Merck will make it possible to prevent severe cases and ease extreme pressure on hospitals, according to the experts


Pharmaceutical companies have raced to produce medication and vaccines to counter the coronavirus pandemic, but until recently only vaccines have shown success in protecting large numbers of people. In the summer of 2020, hopes were pinned on drugs like hydroxychloroquine, until trials eventually showed that it was largely ineffective against the disease, and then remdesivir, which was only able to reduce the length of hospitalization of some patients from 15 to 11 days.
Vaccines have since shown great success where their uptake has been high, as developed countries undertook their biggest inoculation campaigns in history to protect citizens. Hundreds of thousands of lives have been saved, but the limitations are evident: poor countries have hardly received any doses, and even in rich countries significant portions of the population reject vaccines. In immunocompromised patients, vaccines fail to elicit the desired immune response.
As the world prepares to end the second year of the pandemic, the spotlight is once again on medication as an alternative to fight Covid-19. Two antiviral drugs, made by the pharmaceutical giants Pfizer and Merck (which operates as Merck Sharp and Dohme or MSD outside the US and Canada), are now entering the market. Both are being touted as easy to administer and effective in combating the disease.
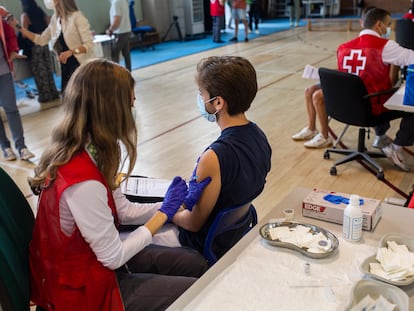
“The fact that the Pfizer and MSD drugs are orally administered is a substantial innovation, because it makes them much easier to use. They slow the progression of the disease at the beginning, when the patient is not yet in a serious condition, and this usually occurs outside a hospital setting,” explained Juan Pablo Horcajada, head of the infectious disease unit at Barcelona’s Hospital Del Mar, and general coordinator of Covid-19 at the hospital.
Pfizer has announced that its antiviral drug, marketed under the brand name Paxlovid, reduces hospitalizations and deaths by 89%, according to its own data. The course of treatment consists of 30 pills to be taken over five days. Ten of these pills contain ritonavir, an old antiviral once used against HIV. The European Medicines Agency (EMA) announced on November 19 that it has begun analyzing Pfizer’s data with the aim of “starting this review to support national authorities who may decide on its early use for Covid-19, for example in emergency use settings, prior to marketing authorisation.” The treatment now awaits a definitive authorization.
The EMA also gave advice on the use of molnupiravir (marketed as Lagevrio), another antiviral drug developed by MSD with Ridgeback Biotherapeutics. That treatment consists of 40 pills to be taken over five days, and it halved the number of cases requiring hospitalization and deaths, according to data provided by both companies.
They slow the progression of the disease at the beginning, when the patient is not yet in a serious condition, and this usually occurs outside a hospital settingJuan Pablo Horcajada, Hospital del Mar, Barcelona
“These two drugs come to fill an important niche as until now we had nothing. There are patients diagnosed in primary care who do not meet the criteria for hospitalization, but who are at risk of the disease subsequently progressing to very severe forms and even death. It was particularly frustrating for us,” explained Santiago Moreno, head of infectious diseases at Madrid’s Ramón y Cajal Hospital. Experts are convinced that the oral administration will help these treatments to reach more people more quickly, as the first five days of symptoms are key. In this way, the two new treatments have a great advantage over other recently developed drugs.
Another category of treatment known as monoclonal antibodies have also demonstrated some success. One is AstraZeneca’s Evusheld, which the pharmaceutical company claims has an efficacy rate of 88%, and which the EMA has been evaluating since October 14. The other is Ronapreve, developed by Regeneron and Roche, which has already received EMA authorization and which helps to treat patients already hospitalized in a serious condition and who lack their own defenses against the coronavirus. “These antibodies are proteins artificially designed to attack a very specific part of the virus and nothing else, which makes them enormously safe, as well as effective,” said Horcajada.
Jesús Sierra, a spokesman for the Spanish Society of Hospital Pharmacy, points out that monoclonal antibodies “are also very important and effective if they are applied in the first five days, but they have two caveats.” The first is that they have to be administered intravenously in the hospital, and secondly that “they are drugs that replace the antibodies that a vaccinated person has. Therefore, they have been tested in the unvaccinated population over 50 years of age. And this population group is very small in Spain.”
In total, the EMA has authorized, is evaluating or has informed member states about more than a dozen treatments against coronavirus. In addition to antivirals and monoclonal antibodies, there are immunosuppressants such as dexamethasone or tocilizumab. “What these drugs do is contain the body’s immune reaction when it is excessive. From a certain moment, which occurs between the fifth and seventh day of the infection, the problem that puts the patient’s life at risk is the excessive reaction of their immune system, and these drugs try to put a stop to that,” explained Horcajada.
The United States has already made agreements with Pfizer and Merck to purchase millions of these new treatments for its citizens, in behavior reminiscent of the Donald Trump’s administration’s hoarding of remdesivir. The US has now acquired 10 million courses of Pfizer’s Paxlovid, with a price tag of $5.3 billion (€4.7 billion), and another 3.1 million courses of Merck’s Lagevrio for around $2.2 billion (€2 billion).
The final stages of development of both drugs have been accompanied by unprecedented announcements from the pharmaceutical giants. Merck said in October it will allow its new drug to be produced as a generic in the 105 lowest-income countries on the planet, and Pfizer made the same announcement for its antiviral last week. None of the major vaccines currently on the market are allowed to be produced as generics.