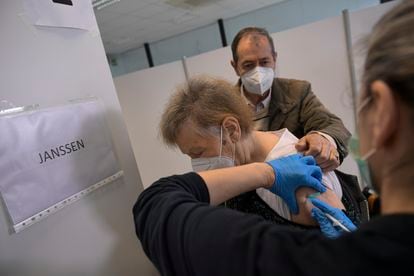
Spanish health authorities opt to use the Janssen vaccine for the 40-49 age group
The campaign will move onto this section of the population during the month of June, but Pfizer and Moderna will be used as an alternative should there be short supply of the one-shot Covid-19 medication
/cloudfront-eu-central-1.images.arcpublishing.com/prisa/L6ALQQGS566AMEGMIAOGTDIJ7Q.jpg)

The Spanish Public Health Commission decided on Tuesday that the Janssen Covid-19 vaccine, which only requires one dose to offer its full protection against serious illness, will be used in Spain for the 40 to 49 age group. The decision by the commission, which is made up of the Health Ministry and the country’s regional health authorities, could see the speed of the vaccination drive accelerate considerably given that a second shot is not required.
Until now, citizens in Spain born from 1972 to 1981 (whatever the month) were going to be given the RNA messenger vaccines – i.e. Moderna and Pfizer-BioNTech. In particular, Pfizer was due to be used given that it has been arriving in Spain in greater quantities than the others.
The decision regarding how to use the vaccine made by Janssen – a company that belongs to pharmaceutical giant Johnson & Johnson – had been put on hold in Spain. The vaccine uses a similar technology as that of the Oxford-AstraZeneca medication, which has had a troubled roll-out in Spain and in other parts of the world. The Spanish authorities have opted not to use AstraZeneca on the under-60s after links with rare blood clots were established. Experts decided that the benefits of using the vaccine on the younger age groups did not outweigh the risks of side effects.
So why isn’t AstraZeneca being used on younger people but Janssen will be? According to what is known up until now, these side effects are much less likely with the use of the latter vaccine. According to data being analyzed by the Spanish Agency of Medicines and Health Products (AEMPS), the frequency of these rare blood clots in the under-60s with AstraZeneca is one in every 200,000 administered doses. In Spain, four deaths have been confirmed to have links to the vaccine after five million doses were administered.
The data for the Janssen vaccine, meanwhile, is based primarily on the experience of the United States, which has reported nine cases of blood clots and one death after having administered 10 million shots – that’s less than a one in a million rate of such side effects.
If the target age group is not lowered below 40, a large proportion of the 17.5 million Janssen shots due to arrive in Spain will be left unused
In Europe, meanwhile, few doses of the Janssen vaccine have been administered, so no statistical conclusions can yet be reached. The latest data being examined by the AEMPS showed that after just under two million doses were administered, there was one case of blood clots, which led to a death. The victim was a 37-year-old woman who received the vaccine in Belgium, according to news agency Reuters. The country’s health authorities decided as a result of the case to limit the vaccine’s use to people aged 41 and over, as they were already doing with the AstraZeneca medication.
So far it has been established that the Covid-19 vaccines that use a viral vector, i.e. Janssen and AstraZeneca, cause these side effects more frequently in younger age groups. This is just the opposite to the effects of Covid-19 itself, which is more serious among older sections of the population. As such, the health authorities are carefully weighing up the lower age limit for the use of these vaccines, seeking the correct balance between the benefits of getting one of these shots and the risks of the rare side effects.
The Public Health Commission’s decision will, however, serve to speed up the vaccination drive, provided that enough doses of the one-shot Janssen vaccine arrive. The initial forecast was for Janssen to send 5.5 million shots in the second quarter of the year, but this will be nigh on impossible now given that just over one million have so far been delivered. Sources from the Health Ministry have explained that the delay is due to an inspection in the US manufacturing plant that caused a shutdown in production. These shortfalls are due to be resolved in July.
The problem is that the longer the deliveries take to arrive, the fewer people who could receive the shot will be left to vaccinate. The initial idea was for the Janssen vaccine to be used among the seven million people in Spain aged between 50 and 59. But 60% of this group has already had their first shot of a Covid-19 vaccine, and the use of Janssen has been scant. In total, just over half-a-million Janssen doses have been used, of which a small proportion has gone to the over-70s.
Some regions, such as the Canary and the Balearic Islands and Castilla-La Mancha, have already started to immunize the 40 to 49 age group, which is the largest sector of the Spanish population with 7.9 million people. The other regions will start to focus on this segment as the month of June progresses. They will start by using the Janssen vaccine but only while supplies last, and the Pfizer and to a lesser extent Moderna medications will be used as an alternative.
In total, Spain is due to receive 17.5 million doses of the Janssen vaccine. If the target age group is not lowered below 40, a large proportion of these shots will be left unused. They will have to either be resold or donated to other countries, given that they will not have arrived on time to be used among the designated age groups.
English version by Simon Hunter.
/cloudfront-eu-central-1.images.arcpublishing.com/prisa/3YE7RPX2HC5PJJULXXNCL2OV24.jpg)
/cloudfront-eu-central-1.images.arcpublishing.com/prisa/2SMTLX7S5HSXUQK6ODWRZV5RRY.jpg)